Abstract
The Nobel Assembly at Karolinska Institutet awarded the 2021 Nobel Prize in Physiology and Medicine jointly to David Julius and Ardem Patapoutian for their discoveries of receptors for temperature and touch. TRP and Piezo channels also have several additional physiological functions, so targeting their functions could be a promising therapeutic target for different diseases, including the management of pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Our ability to sense heat, cold, and touch is crucial for survival and to interact with the surrounding world. This year’s Nobel Prize laureates identified the missing connections to understand the difficult interaction between our senses and the environment [1].
Nobel recognition
David Julius (1955–, University of California, San Francisco, CA, USA) utilized capsaicin, a spicy compound from chili peppers to identify a sensor in the nerve endings of the skin that responds to heat (Fig. 1) [1].
Ardem Patapoutian (1967–, Scripps Research in La Jolla, California, USA) used pressure-sensitive cells to discover a novel class of sensors that respond to mechanical stimuli in the skin and internal organs (Fig. 2) [1].
David Julius and heat sensation
A major focus of his work has been to identify and understand molecular mechanisms involved in our senses of touch and pain. His group has exploited the properties of natural products to discover a family of temperature-sensitive ion channel receptors (transient receptor potential channels, TRP, ‘trip’) that make possible the sensory nerve fibres to detect hot or cold temperatures and also respond to chemical stimuli. In 2013, Julius and UCSF colleague Yifan Cheng, PhD, used electron microscopy to determine the structure of the TRPV1 receptor at near-atomic resolution. In 2015, Julius and Cheng used to same techniques to determine the structure of TRPA1, the so-called “wasabi receptor” [2].
The discovery of TRPV1 was a major breakthrough leading the way to the recognisation of additional temperature-sensing receptors [1].
Independently of one another, both David Julius and Ardem Patapoutian used the chemical substance menthol to identify TRPM8, a receptor that was shown to be activated by cold or menthol [1].
Altogether, natural products (irritants from pepper, mustard plants) have revealed three members of the TRP ion channel family: TRPV1, TRPM8, and TRPA1. They are molecular detectors of thermal and chemical stimuli that activate sensory neurons to produce pain. Analysis of TRP channel function and expression has proved the existence of nociceptors as a specialized group of somatosensory [3, 4].
TRPs are multifunctional signalling molecules, expressed in many tissues and cell types. Most TRPs are can be activated by both physical (temperature, voltage, pressure, and tension) and chemical stimuli. Some TRPs function as non-selective cation channels in the plasma membrane; others regulate Ca2+ release in intracellular organelles [5].
The mammalian TRP channel superfamily has 28 members (27 in humans). The superfamily is divided into six subfamilies: canonical (short TRPs, TRPC1 − 7), vanilloid (TRP channel subfamily V, TRPV1 − 6), melastatin (TRP channel subfamily M, TRPM1 − 8), ankyrin (TRP channel subfamily A, TRPA1), mucolipins (TRPML1 − 3), and polycystins (polycystic kidney disease 2-like 1 protein (PKD2L1, also termed TRPP3) and polycystin-2 (TRPP2)) [5].
Mutations in genes encoding TRP channels are the cause of several inherited diseases in humans (the so-called ‘TRP channelopathies’) that affect the cardiovascular, renal, skeletal, and nervous systems. Recent reports showed that two mutations in TRPM3 are associated with a developmental and epileptic encephalopathy, pointing to an important role of TRPM3 in the human brain [6].
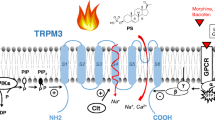
Medical control of chronic pain is frequently unsatisfactory. Opioids are effective painkillers, but they are also addictive, causing side effects. Most TRP channels are located at the cell surface, which makes them generally accessible drug targets, to target the beginning of the pain to avoid the side effects of opioids. A number of potent, small-molecule TRPV1, TRPV3, and TRPA1 antagonists have already entered clinical trials as novel analgesic agents. The researches with TRP channels expand into new clinical indications ranging from respiratory disorders (asthma) through neurological and psychiatric diseases (anxiety) to diabetes, obesity, metabolic disorders, cancer, and asthma. A better understanding of TRP channel functions in health and disease should lead to the discovery of first-in-class drugs for these unmanageable diseases [2, 7] (Fig. 3).
New targets of analgesics [8]
Arden Patapoutian and mechanical sensation
Ardem Patapoutian worked to identify the mechanical receptors that are activated by mechanical stimuli. Patapoutian used cultured mechanosensitive cells to identify an ion channel activated by mechanical force. Patapoutian and his co-workers succeeded in identifying a single gene whose silencing provided the cells insensitive to pressure with the micropipette. A new mechanosensitive ion channel had been discovered and was given the name Piezo1, after the Greek word for pressure. A second gene was discovered and named Piezo2. Sensory neurons were found to express high levels of Piezo2. Piezo1 and Piezo2 are ion channels that are directly activated by the exertion of pressure on cell membranes, are involved in cell mechanotransduction — the conversion of mechanical forces into biological signals. Piezos are excitatory channels, with their activation (touch, muscular tension, shear stress) producing membrane depolarization. Piezo channel openings lead to Ca2+ entry into the cell, potentially triggering intracellular Ca2+ signalling pathways [1, 9].
Piezo2 ion channel is not only essential for the sense of touch; moreover, Piezo2 was shown to play an important role in proprioception, sensing of body position, and motion. Piezo1 and Piezo2 channels have been shown to regulate additional important physiological processes such as allodynia, baroreceptor reflex, developmental processes (such as lymphatic valve development, heart valve development, angiogenesis, and stem cell differentiation), and regulatory processes (such as bone formation, cell migration, axon regeneration, the inflammatory response of innate immune cells, and red blood cell (RBC) volume regulation) affecting erythrocyte volume regulation, cell division, innate immunity, cardiovascular regulation, control of blood pressure, respiration, and urinary bladder [10] (Fig. 4).
Localization, biological function of Piezo channels [11]
Several mutations in Piezo channels have been shown to cause multiple hereditary human disorders, such as autosomal recessive congenital lymphatic dysplasia, hereditary xerocytosis, and an autosomal recessive syndrome of muscular atrophy with perinatal respiratory distress. Mutations that cause dehydrated hereditary xerocytosis to alter the rate of Piezo channel inactivation, indicating the critical role of their kinetics in normal physiology [11].
Despite the large size of Piezo proteins, relatively few pharmacological tools have been found to modulate their activity (tarantula toxin, Yoda1, Jedi ½, Dooku1) [11].
Summary
The discoveries of the TRPV1, TRPM8, and Piezo channels by 2021 Nobel Prize laureates have allowed us to understand how heat, cold, and mechanical force can initiate the nerve impulses that make able us to perceive and adapt to the world around us. The TRP channels are important to perceive temperature. The Piezo2 channel enables us for the sense of touch and to feel the position and movement of our body parts. TRP and Piezo channels also have several additional physiological functions. Further researches are ongoing to develop treatments for several disease conditions, including chronic pain, targeting TRP and Piezo channels.
Availability of data and material
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
https://www.nobelprize.org/prizes/medicine/2021/press-release/
https://www.ucsf.edu/news/2021/09/421486/biography-david-julius
Julius D (2013) TRP channels and pain. Annu Rev Cell Dev Biol 29:355–384
Roper SD (2014) TRPs in taste and chemesthesis. Handbook Experimen Pharmacol 223:827–871
Koivisto AP, Belvisi MG, Gaudet R, Szallasi A (2021) Advances in TRP channel drug discovery: from target validation to clinical studies. Nat Rev Drug Discov 15:1–19
Zhao S, Rohacs T (2021) The newest TRP channelopathy: gain of function TRPM3 mutations cause epilepsy and intellectual disability. Channels (Austin) 15:386–397
Fernández-Carvajal A, Fernández-Ballester G, Devesa I, González-Ros JM, Ferrer-Montie A (2012) New strategies to develop novel pain therapies: addressing thermoreceptors from different points of view. Pharmaceuticals 5:16–48
Saleem M, Naz H (2017) Analgesics: new target and sources 10:67853. https://www.intechopen.com/chapters/55367
Parpaite T, Coste B (2017) Piezo channels. Curr Biol 27:R250–R252
Kefauver JM, Ward AB, Patapoutian A (2020) Discoveries in structure and physiology of mechanically activated ion channels. Nature 587:567–576
Fang XZ, Zhou T, Xu JX, Wang YX (2021) Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci 11(1). https://doi.org/10.1186/s13578-020-00522-z
Funding
Open access funding provided by Semmelweis University.
Author information
Authors and Affiliations
Contributions
H.K.: collecting background data, writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hagymási, K. The nobel prize in physiology or medicine — 2021. Struct Chem 33, 987–990 (2022). https://doi.org/10.1007/s11224-022-01890-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-01890-7