Abstract
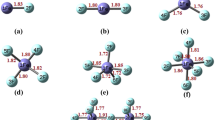
Novel strong superacids HMF6 (M=Au, Ir, Os, Re, Ta, W) are proposed and are investigated with the help of DFT/B3LYP method and SDD basis set for 5d transition metals as well as 6-311++G (d) basis set for H and F atoms. These HMF6 superacids are composed with Brønsted/Lewis (MF5/HF). The stabilities of HMF6 are discussed with the help of structure, dissociation energy through HF channel, and normal mode analysis. The ΔEdisso>0 shows that all HMF6 superacids are energetically stable through HF dissociation channel. The gas phase acidity of HMF6 has been calculated by the Gibbs free deprotonation energy. All species of HMF6 belong to superacids having smaller deprotonation energy; 100% concentrated H2SO4 acids however predicted ΔGdep of HAuF6, is nearly equal to ΔGdep of HSbF6. The strength of acidity of HMF6 is closely related to vertical detachment energy (VDE) of their corresponding superhalogen anions \( \kern0.5em {\mathrm{MF}}_6^{-} \). This study provide appropriate path to design new class of superacids which is more acidic than HSbF6. We have also modelled and discussed supersalt by the interaction of Li with MF6 superhalogen.






Similar content being viewed by others
Data availability
Data and materials are real. First time any study is done on these aforesaid materials.
References
Nishikawa K, Nojima H (2001) Japan J. Appl. Phys. 40:835–837
Miller NJ (1984). J. Ment. Health Adm 11:36–37
Giri S, Bahera S, Jena P (2014) Angew Chem. Int. Ed 53:13916–13919
Srivastava AK, Misra N (2016). Polyhedron 117:422–426
Srivastava AK, Misra N (2016) Electrochem. Commun 68:99–103
Koppel IA, Burk P, Leito I, Sonoda T, Mishima M (2000). J. Am. Chem. Soc. 122:5114–5124
Hall NF, Conant JB (1927). J. Am. Chem. Soc. 49:3047–3061
Hogeveen H, Bickel AF (1969) Recl. Trav. Chim. Pays-Bas 88:371–378
Hogeveen H, Bickel AF (1967) J. Chem. Soc. Chem. Commun. 13:635–636
Gillespie RJ, Peel TE (1971) Adv. Phys. Org. Chem. 9:1–24
Gillespie RJ, Peel TE (1973) J. Am. Chem. Soc. 95:5173–5178
Olah GA, Schlosberg RH (1968) J. Am. Chem. Soc. 90:2726–2727
Bickel AF, Gaasbeek CJ, Hogeveen H, Oelderik JM, Platteeuw JC (1967) Chem Commun 634–635
Olah GA, Lukas J (1967). J. Am. Chem. Soc. 89:2227–2228
Olah GA, Prakash GK, Sommer J (1979) Superacids. Science 206:13–20
Czapla M, Skurski P (2015). Chem. Phys. Lett. 630:1–5
Czapla M, Skurski P (2015). J. Phys. Chem. A 119:12868–12875
Srivastava AK, Misra N (2015). Polyhedron 3:277–283
Shukla DV, Srivastava AK, Misra N (2017). Main group chemistry 16(2):141–150
Czapla M, Skurski P, Anusiewicz I (2016). RSC Adv. 6:29314–29325
Srivastava A K, Misra N Kumar A (2017) New J Chem 41:5445–5449
Frisch, MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision E.01, Gaussian, Inc., Wallingford CT, 2009
Topol IA, Tawa GJ, Burt SK, Rashin AA (1997). J. Phys. Chem. A 101:10075–10081
Dennington R, Keith T, Millam J (2009) GaussView Version 5, Semichem Inc. Shawnee Mission KS
Macgregor SA, Moock KH (1998). Inorg. Chem. 37:3284
Graudejus O, Wilkinson A P, Chacon L C, Bartlett N (2000) Inorg Chem 39(13):2794–2800
Hoskins BF, Linden A, Mulvaney PC, O’Donnell TA (1984). Inorg.Chim. Acta 88:217
Fitz H, Muller BG, Graudejus O, Bartlett NZ (2002). Z. Anorg. Allg. Chem. 628:133
Graudejus O, Muller BGZ (1996). Z. Anorg. Allg. Chem. 622:1076
George PM, Beauchamp JL (1979). Chem. Phys. 36:345
Viggiano AA, Paulson JF, Dale F, Henchman M, Adams NG, Smith DJ (1985). Phys. Chem. 89:2264
Korobov MV, Kuznetsov SV, Sidorov LN, Shipachev VA, Mit’kin VN (1989). Int. J. Mass Spectrom. Ion Process. 87:13
Friedman JF, Stevens AE, Miller TM, Viggiano AA (2006). J. Chem. Phys. 124:224306
Craciun R, Picone D, Long RT, Li S, Dixon DA (2010). Inorg. Chem. 49:1056–1070
Gutsev GL, Boldyrev AI (1983). Chem. Phys. Lett. 101:441
Miyoshi E, Sakai Y, Murakami A, Iwaki H, Terashima H, Shoda T, Kawaguchi T (1988). J. Chem. Phys. 89:4193
Miyoshi E, Sakai Y (1988). J. Chem. Phys. 89:7363
Koirala P, Willis M, Kiran B, Kandalam AK, Jena P (2010). J. Phys. Chem. C 114:16018–16024
Srivastava AK, Misra N, Pandey SK (2015). Chem. Phys. Lett. 624:15–18
Siddiqui SA, Rasheed T (2012) Int J Quantum Chem 113(7):959–965
Srivastava AK, Misra N (2014). J. Fluor. Chem. 158:65–68
Srivastava AK, Misra N, Pandey AK (2015). J. Chem. Sci. 127:1853–1858
Czapla M, Skurski P (2017). Int. J. Quantum Chem. 25:494
Koch U, Popelier P (1995). J. Phys. Chem. A 99:9747–9754
O’boyle NM, Tenderholt AL, Langner KM (2008). J. Comput. Chem. 29:839–845
Keith T A, Gristmill T K Software overland park KS USA 2019.
Rasheed T, Siddiqui SA, Pandey AK, Bouarissa N, AliAl-Hajry (2017). J. Fluor. Chem. 195:85–92
Rasheed T, Siddiqui SA, Pandey AK, Mishra M (2012). J. Fluor. Chem. 135:285–291
Code availability
Licenced codes are used.
Author information
Authors and Affiliations
Contributions
Anoop Kumar Pandey: Most calculations and writing.
D. V. Shukla: Modelling and writing.
Vijay Narayan: Literature survey and writing.
Vijay Singh: Methods and some calculations.
Apoorva Dwivedi: Whole paper final writing and submission process (corresponding author).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOC 312 kb)
Rights and permissions
About this article
Cite this article
Pandey, A.K., Shukla, D.V., Narayan, V. et al. Protonated MF– (M=Au, Ir, Os, Re, Ta, W) behave as superacids and are building blocks of new class of salt. Struct Chem 33, 91–100 (2022). https://doi.org/10.1007/s11224-021-01809-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-021-01809-8