Abstract
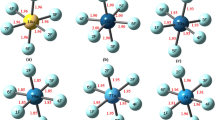
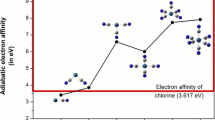
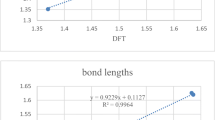
The superacidic properties of HFemFn (m = 1/2, n = 1–6/11) are calculated by using the Density functional theory with Becke, 3-parameter, Lee–Yang–Parr (DFT/B3LYP) method. To improve accuracy, the Los Alamos National Laboratory 2 double-ζ (LAN2DZ) basis set is employed for Fe and 6-31G (d) basis set for H and F atoms in the calculations. The gas phase acidity of HFemFn (m = 1/2, n = 1–6/11) has been calculated by using Gibbs free energies of their deprotonation reactions. The calculated correlation factor (R2 = 0.97452) shows that the acidity of protonated superhalogens HFemFn (m = 1/2, n = 1–6/11) is directly related to the stability of their respective anionic superhalogens. HFeF6 acid has comparable strength, while HFe2F11 acid is stronger than the most acidic species HSbF6. The current study establishes a novel approach for modeling new superacids that are more acidic than the strongest superacid, HSbF6. We have also designed various supersalts using a combination of superacids HFeF3 (odd n) HFeF4 (even n), and superbase OLi3OH. The calculated dissociation energies through the neutral and ionic channels of supersalts are compared with the respective dissociation energies of traditional salt LiF. In supersalts, the computed nonlinear optical properties (NLO) are affected by whether the number of fluorine atoms is even or odd.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Nishikawa K, Nojima H (2001) Jpn J Appl Phys 40:L835–L837
Miller NJ, Ment J (1984) Health Adm 11:36–37
Goel N, Etwaroo GR (2006) Psychol Med 36:1253–1263
Rienstra-Kiracofe JC, Tschumper GS, Schaefer III HF, Nandi S, Ellison GB (2002) Chem. Rev. (Wasington.D.C) 102: 231–282
Gutsev GL, Boldyrev AI (1984) Chem Phys Lett 108:250–254
Röper M, Gehrer E, Narbeshuber T, Siegel W (2000) “Acylation and Alkylation” in Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim
Stichert W, Scuth F, Kuba S, Knozinger H (2001) J Catal 198:277–285
Harmer MA, Junk C, Rostovtsev V, Carcani LG (2007) Vickery, Schnepp Z. Green Chem 9:30–37
Zhou FQ, Xu WH, Li JF et al (2017) Inorg Chem 56:11787–11797
Pines H (1981) J Am Chem Soc 119:8576–8577
Hall NF, Conant JB (1927) J Am Chem Soc 49:3047–3061
Gillespie RJ, Peel TE (1971) Adv Phys Org Chem 9:1–24
Gillespie RJ, Peel TE (1973) J Am Chem Soc 95:5173–5178
Hogeveen H, Bickel AF (1969) Recl Trav Chim Pays-Bas 88:371–378
Hogeveen H, Bickel AF (1967) J Chem Soc, Chem Commun 13:635–636
Bickel AF, Gaasbeek CJ, Hogeveen H, Oelderik JM, Platteeuw JC (1967) J Chem Soc, Chem Commun 13:634–635
Olah GA, Lukas J (1967) J. Am. Chem. Soc. 89:2227–2228
Olah GA, Prakash GK, Sommer J (1979) Science 206:13–20
Koppel IA, Burk P, Koppel I, Leito I, Sonoda T, Mishima M (2000) J Am Chem Soc 122:5114–5124
Czapla M, Skurski P (2015) Chem Phys Lett 630:1–5
Czapla M, Skurski P (2015) J Phys Chem A 119:12868–12875
Srivastava AK, Misra N (2015) Polyhedron 3:277–283
Zhong G, Chan B, Radom L (2009) Org Lett 11:749–751
Gutowski KE, Dixon DA (2006) J Phys Chem A 110:12044–12054
Axhausen J, Ritter C, Lux K, Kornath A (2013) Anorg Allg Chem 639:65–72
Axhausen J, Lux K, Kornath A (2014) Angew Chem Int Ed 53:3720–3721
Yang H, Li Y, Wu D, Li ZR (2012) Int J Quantum Chem 112:770–778
Srivastava AK, Misra N (2014) New J Chem 38:2890–2893
Srivastava AK, Misra N (2014) Mol Phys 112:2621–2626
Srivastava AK, Misra N (2014) RSC Adv 4:41260–41265
Becke BD (1993) J Chem Phys 98:5648–5652
Frisch MJ et al.(2003) Gaussian 03 Revision A. 1, Gaussian, Inc., Pittsburgh PA
Dennington R, Keith T, Millam J (2005) GaussView Version 30. Semichem Inc, KS
Rasheed T, Siddiqui SA, Pandey AK, Bouarissae N, Hajry AA (2017) J Fluorine Chem 195:85–92
Topol IA, Tawa GJ, Burt SK, Rashin AA (1997) J Phys Chem A 101:10075–10081
Czapla M, Skurski P (2017) Int J Quantum Chem 118:25494–25498
Pandey AK, Shukla DV, Mishra VN, Singh V, Dwivedi A, Struct Chem 33:91–100
Srivastava AK, Kumar A, Mishra N (2017) New Jour. Chem 41:5445–5449
Pandey AK, Shukla DV, Mishra VN, Dwivedi A (2021) Jour Ind chem Soc 98:100046
Rasheed T, Siddiqui SA, Kargeti A, Shukla DV, Singh V, Pandey AK (2021) Stru Chem 32:2209–2221
Srivastava AK, Misra N (2016) Chem Phys Lett 644:1–4
Padmanabhan J, Parthasarathi R, Subramanian V, Chattaraj PK (2007) J Phys Chem A 111:1358–1361
Yang H, Li Y, Wu D, Li ZR (2012) Inter Jour Quant Chem 112:770–778
Yang H, Li Y, Wu D, Li ZR (2008) Inorg Chem 47:9773–9778
Padrón JA, Carasco R, Pellón RF (2002) J Pharm Pharmaceut Sci 5:258–266
Verma RP, Hansch C (2005) Bioorg Med Chem 13:2355–2372
Verma RP, Kurup A, Hansch C (2005) Bioorg Med Chem 13:237–255
Vuks MF (1966) Opt Spectrosc 20:361–368
Kumar A, Srivastava A, Tiwari SN, Misra N, Sharma D (2019) Mol Cryst Liq Cryst 681:23–31
Acknowledgements
VS is grateful and acknowledge the computer resources, technical expertise, and assistance provided by the Center for High-Performance Computing (MATS1467) Cape Town, South Africa.
Funding
Funding is provided by Uttar Pradesh government (India)[No:46/2021/603/sattar-4–2021-4(56)/2020] to the author Anoop Kumar Pandey.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by all the authors. The first draft of the manuscript was written by Anoop Kumar Pandey, Apoorva Dwivedi, and Vijay Singh and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies involving animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandey, A.K., Dwivedi, A., Shukla, D.V. et al. Investigation of superacidic behavior of hydrogenated FemFn (m = 1/2, n = 1–6/11) complexes and their abilities to form supersalts. Struct Chem 34, 1385–1393 (2023). https://doi.org/10.1007/s11224-022-02099-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-02099-4