Abstract
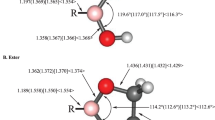
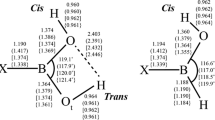
Results of structural and thermochemical calculations involving boronic acid, HB(OH)2, and the corresponding ethylene glycol ester, HB(-O-CH2-CH2-O-), in the presence of explicit NH3 and/or H2O molecules are reported. Calculations were performed in a polarizable continuum model (PCM) water solution and in the gas phase using density functional theory (DFT) and second-order Moller-Plesset perturbation theory (MP2) with the Dunning-Woon aug-cc-pVTZ basis set. Different classes of local minima on the HB(OH)2·NH3·H2O and HB(-O-CH2-CH2-O-)·NH3·H2O potential energy surfaces (PESs) in PCM water solution have been identified: (1) structures with a N→B dative bond, [H3N→BH(OH)2]·H2O, and [H3N→B(H)(-O-CH2-CH2-O-)]·H2O, where the H2O is involved in hydrogen bonding; (2) water-inserted structures involving either a novel O→B dative bond, H3N·H(H)O→BH(OH)2, and H3N···H(H)O→B(H)(-O-CH2-CH2-O-) where the H2O molecule remains essentially intact or lower-energy zwitterionic arrangements in which a water H atom has been transferred to the ammonia, [H4N]+[HO-BH(OH)2]−, and [H4N]+[BH(OH)(-OCH2-CH2-O-)]−; (3) structures where both the NH3 and H2O molecules are exclusively involved in hydrogen bonding. In these simple model systems, arrangements with N→B dative bonds, and some structures with only O···H and N···H hydrogen bonds, are ca. 5–6 kcal/mol lower in energy than either of the corresponding water-inserted structures.





Similar content being viewed by others
References
Jin S, Cheng Y, Reid S, Li M, Wang B (2010) Carbohydrate recognition by boronolectins, small molecules, and lectins. Med Res Rev 30:171–257
Yang W, Gao S, Gao X, Karnati VV, Ni W, Wang B, Hooks WB, Carson J, Weston B (2002) Diboronic acids as fluorescent probes for cells expressing Sialyl Lewis X. Bioorg Med Chem Lett 12:2175–2177
Murrey HE, Hsieh-Wilson LC (2008) Chemical neurobiology of carbohydrates. Chem Rev 108:1708–1731
Sun X, Zhai W, Fossey JS, James TD (2016). Boronic acids for fluorescence imaging of carbohydrates. Chem Commun 52:3456–3469
James TD, Sandanayake KRAS, Nakashima K, Shinkai S (1994) A glucose-selective molecular fluorencence sensor. Chem Commun 1621–1622
James TD, Sandanayake KRAS, Shinkai S (1995) Chiral discrimination of monosaccharides usin a fluorescent molecular sensor. Nature 374:345–347
Ni W, Kaur G, Springsteen G, Wang B, Franzen S (2004) Regulating the fluorescence intensity of an anthracene boronic acid system: a B-N bond or a hydrolysis mechanism? Bioorg Chem 32:571–581
Chapin BM, Metola P, Vankayala SL, Woodcock HL, Mooibroek TJ, Lynch VM, Larkin JD, Anslyn EV (2017) Disaggregation is a mechanism for emission turn-on of ortho-aminomethylphenylboronic acid-based saccharide sensors. J Am Chem Soc 139:5568
Sun X, James TD, Anslyn EV (2018) Arresting “loose bolt” internal conversion from −B(OH)2 groups is the mechanism for emission turn-on in ortho-aminomethylphenylboronic acid-based saccharide sensors. J Am Chem Soc 140:2348–2354
Sun X, Chapin BM, Metola P, Collins B, Wang B, James TD, Anslyn EV (2019) The mechanisms of boronate ester formation and fluorescent turn-on in ortho-aminomethylphenylboronic acids. Nat Chem 11:768–778
Ernzerhof M, Perdew JP (1998) Generalized gradient approximation to the angle- and system-averaged exchange hole. J Chem Phys 108:3313–3320
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Perdew JP, Burke K, Ernzerhof M (1997) Errata: generalized gradient approximation made simple. Phys Rev Lett 1396
Dunning Jr TH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023
Kendall RA, Dunning Jr TH, Harrison RJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys 96:6796–6806
Peterson KA, Woon DE, Dunning Jr TH (1994) Benchmark calculations with correlated molecular wave functions. IV. The classical barrier height of the H + H2→ H2 + H reaction. J Chem Phys 100:7410–7415
Woon DE, Dunning Jr TH (1993) Gaussian basis sets for use in correlated molecular calculations. III The atoms aluminum through argon. J Chem Phys 98:1358–1371
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR al., e. Gaussian 09 Revision D.01
Tomasi J, Mennucci B, Cammi T (2005) Quantum mechanical continuum solvation models. Chem Rev 105(8):2999–3094
Grimme S, Antony J, Erlich S, Krieg H (2010) A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104
Grimme S, Ehrlich S, Goerigk L (2011) Effect of damping function in dispersion corrected density functional theory. J Comput Chem 32(7):1456–1465
Frisch MJ, Head-Gordon M, Pople JA (1990) A direct MP2 gradient method. Chem Phys Lett 166:275–280
Head-Gordon M, Pople JA, Frisch MJ (1988) MP2 energy evaluation by direct methods. Phys Lett 153:503–506
Moller C, Plesset MS (1934) Note on the approximation treatment for many-electron systems. Phys Rev 46:0618–0622
Rao NZ, Larkin JD, Bock CW (2016) A comparison of the structure and bonding in the aliphatic boronic R-B(OH)2 and borinic R-BH(OH) acids (R=H, NH2, OH, and F): a computational investigation. Struct Chem 27:1081–1091
Buhl M, Steinke T, Schleyer P v R, Boese R (1991) Solvation effects on geometry and chemical shifts. An ab initio study. Angew Chem Int Ed Eng 30:1160
Hopfl H (1999) The tetrahedral character of the boron atom newly defined - a useful tool to evaluate the N-->B bond. J Organomet Chem 581:129–149
Jiao H, Schleyer P (1994) Large effects of medium on geometries. An ab Initio Study. v. R. J Am Chem Soc 116:7429
Larkin JD, Bock CW (2018) A comparison of the structure and bonding in the donor-acceptor complexes H3N→BR(OH)2 and H3N→BRH(OH) (R = H; NH2, OH, and F): a computational investigation. Struct Chem 30:361–368
Larkin JD, Fossey JS, James TD, Brooks BR, Bock CW (2010) A comparison of the structure and bonding in the donor-acceptor complexes H3N→BR(OH)2 and H3N→BRH(OH) (R = H; NH2, OH, and F): a computational investigation. J Phys Chem A 114:12531
Collins BE, Sorey S, Hargrove AE, Shabbir SH, Lynch VM, Anslyn EV (2009) Probing intramolecular B-N interactions in ortho-aminomethyl arylboronic acids. J Organomet Chem 11:4055–4060
Zhu L, Shabbir SH, Gray M, Lynch VM, Sorey S, Anslyn EV (2006) A structural investigation of the N-B interaction in an o-(N,N-dialkylaminomethyl)arylboronate system. J Am Chem Soc 128:1222–1232
Franzen S, Ni W, Wang B (2003) Study of the mechanism of electron-transfer quenching by boron−nitrogen adducts in fluorescent sensors. J Phys Chem B 107:12942–12948
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 52.1 kb)
Rights and permissions
About this article
Cite this article
Markham, G.D., Larkin, J.D. & Bock, C.W. Models for boronic acid receptors: a computational structural, bonding, and thermochemical investigation of the HB(OH)2∙H2O∙NH3 and HB(-O-CH2-CH2-O-)∙NH3∙H2O potential energy surfaces. Struct Chem 32, 607–621 (2021). https://doi.org/10.1007/s11224-020-01701-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-020-01701-x