Abstract
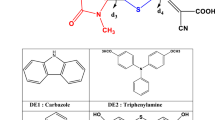
The functionality of the proton-coupled electron transfer (PCET) model was tested on a squaraine-sensitized solar cell. The geometrical parameters, excitations, and electronic structures of free and Ti+4-bound squaraine dye were monitored using a set of pure and hybrid density functional theory (DFT) functionals with diffuse and polarization functions. The infrared spectra showed the dye-metal proton transfer. The UV-Vis spectra of unbound and bound squaraine dye using the pure functional (PBEPBE) are in excellent agreement with the experimental ones. The first photoexcited state charge transfer enhanced the charge density around the anchoring group of neat and bound squaraine dye. The injection of electronic charge into the titanium complex was confirmed by density of states (DOS) and natural bond orbital (NBO) analyses. The comparatively high total hyperpolarizability of the squaraine dye is indicative of a potent nonlinear optical (NLO) devise.









Similar content being viewed by others
References
Green MA (1993) Silicon solar cells: evolution, high-efficiency design and efficiency enhancements. Semicond Sci Technol 8(1):1
Overstraeten RV, Mertens R, Nijs J (1982) Progress in photovoltaic energy conversion. Rep Prog Phys 45(10):1041
Rolf B (2001) Review of layer transfer processes for crystalline thin-film silicon solar cells. Jpn J Appl Phys 40(7R):4431
Jacoby M (2016) The future of low-cost solar cells. Chem Eng News 94:30–35
O'Regan B, Gratzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353(6346):737–740
Balasingam SK, Lee M, Kang MG, Jun Y (2013) Improvement of dye-sensitized solar cells toward the broader light harvesting of the solar spectrum. Chem Commun 49(15):1471–1487. https://doi.org/10.1039/C2CC37616D
Jung HS, Lee J-K (2013) Dye sensitized solar cells for economically viable photovoltaic systems. The Journal of Physical Chemistry Letters 4(10):1682–1693. https://doi.org/10.1021/jz400112n
Kloo L (2013) On the early development of organic dyes for dye-sensitized solar cells. Chem Commun 49(59):6580–6583. https://doi.org/10.1039/C3CC42733A
Nazeeruddin MK, Baranoff E, Grätzel M (2011) Dye-sensitized solar cells: a brief overview. Sol Energy 85(6):1172–1178. https://doi.org/10.1016/j.solener.2011.01.018
Fischer MKR, Wenger S, Wang M, Mishra A, Zakeeruddin SM, Grätzel M, Bäuerle P (2010) D-π-a sensitizers for dye-sensitized solar cells: linear vs branched oligothiophenes. Chem Mater 22(5):1836–1845. https://doi.org/10.1021/cm903542v
Zhigang C, Fuyou L, Chunhui H (2007) Organic D-A dyes for dye-sensitized solar cell. Curr Org Chem 11(14):1241–1258. https://doi.org/10.2174/138527207781696008
Ambrosio F, Martsinovich N, Troisi A (2012) Effect of the anchoring group on electron injection: theoretical study of phosphonated dyes for dye-sensitized solar cells. J Phys Chem C 116(3):2622–2629. https://doi.org/10.1021/jp209823t
Chen S-L, Yang L-N, Li Z-S (2013) How to design more efficient organic dyes for dye-sensitized solar cells? Adding more sp2-hybridized nitrogen in the triphenylamine donor. J Power Sources 223:86–93. https://doi.org/10.1016/j.jpowsour.2012.09.053
Feng J, Jiao Y, Ma W, Nazeeruddin MK, Grätzel M, Meng S (2013) First principles design of dye molecules with ullazine donor for dye sensitized solar cells. J Phys Chem C 117(8):3772–3778. https://doi.org/10.1021/jp310504n
Katono M, Bessho T, Wielopolski M, Marszalek M, Moser J-E, Humphry-Baker R, Zakeeruddin SM, Grätzel M (2012) Influence of the anchoring modes on the electronic and photovoltaic properties of D−π–a dyes. J Phys Chem C 116(32):16876–16884. https://doi.org/10.1021/jp304490a
Mohammadi N, Mahon PJ, Wang F (2013) Toward rational design of organic dye sensitized solar cells (DSSCs): an application to the TA-St-CA dye. J Mol Graph Model 40:64–71. https://doi.org/10.1016/j.jmgm.2012.12.005
Ning Z, Fu Y, Tian H (2010) Improvement of dye-sensitized solar cells: what we know and what we need to know. Energy Environ Sci 3(9):1170–1181. https://doi.org/10.1039/C003841E
Yum J-H, Walter P, Huber S, Rentsch D, Geiger T, Nüesch F, De Angelis F, Grätzel M, Nazeeruddin MK (2007) Efficient far red sensitization of nanocrystalline TiO2 films by an unsymmetrical squaraine dye. J Am Chem Soc 129(34):10320–10321. https://doi.org/10.1021/ja0731470
Huynh MHV, Meyer TJ (2007) Proton-coupled electron transfer. Chem Rev 107(11):5004–5064. https://doi.org/10.1021/cr0500030
Mayer JM (2004) Proton-coupled electron transfer: a reaction chemist’s view. Annu Rev Phys Chem 55(1):363–390. https://doi.org/10.1146/annurev.physchem.55.091602.094446
Cukier RI, Nocera DG (1998) Proton-coupled electron transfer. Annu Rev Phys Chem 49(1):337–369. https://doi.org/10.1146/annurev.physchem.49.1.337
Hammes-Schiffer S (2001) Theoretical perspectives on proton-coupled electron transfer reactions. Acc Chem Res 34(4):273–281. https://doi.org/10.1021/ar9901117
Rosenthal J, Nocera DG (2007) Role of proton-coupled electron transfer in O–O bond activation. Acc Chem Res 40(7):543–553. https://doi.org/10.1021/ar7000638
Hammes-Schiffer S, Soudackov AV (2008) Proton-coupled electron transfer in solution, proteins, and electrochemistry. J Phys Chem B 112(45):14108–14123. https://doi.org/10.1021/jp805876e
Luo S, Zhang E, Su Y, Cheng T, Shi C (2011) A review of NIR dyes in cancer targeting and imaging. Biomaterials 32(29):7127–7138. https://doi.org/10.1016/j.biomaterials.2011.06.024
Law KY, Bailey FC (1987) Squaraine chemistry: effect of synthesis on the morphological and xerographic properties of photoconductive squaraine. Journal of Imaging Science 31(4):172–177
Ajayaghosh A (2005) Chemistry of Squaraine-derived materials: near-IR dyes, low band gap systems, and cation sensors. Acc Chem Res 38(6):449–459. https://doi.org/10.1021/ar0401000
Jipson VB, Jones CR (1981) Infrared dyes for optical storage. J Vac Sci Technol 18(1):105–109. https://doi.org/10.1116/1.570684
Law KY (1987) Squaraine chemistry: effects of structural changes on the absorption and multiple fluorescence emission of bis[4-(dimethylamino)phenyl]squaraine and its derivatives. J Phys Chem 91(20):5184–5193. https://doi.org/10.1021/j100304a012
Law K-Y (1995) Squaraine chemistry. Absorption, fluorescence emission, and photophysics of unsymmetrical Squaraines. J Phys Chem 99(24):9818–9824. https://doi.org/10.1021/j100024a024
Xu W, Peng B, Chen J, Liang M, Cai F (2008) New triphenylamine-based dyes for dye-sensitized solar cells. J Phys Chem C 112(3):874–880. https://doi.org/10.1021/jp076992d
Wong BM, Cordaro JG (2008) Coumarin dyes for dye-sensitized solar cells: a long-range-corrected density functional study. J Chem Phys 129(21):214703. https://doi.org/10.1063/1.3025924
Peng B, Yang S, Li L, Cheng F, Chen J (2010) A density functional theory and time-dependent density functional theory investigation on the anchor comparison of triarylamine-based dyes. J Chem Phys 132(3):034305. https://doi.org/10.1063/1.3292639
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian, Inc., Wallingford
Dennington R, Keith T, Millam J (2009) GaussView. Version 5, vol Shawnee Mission. Semichem. Inc.
Chemissian. http://www.chemissian.com/
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592. https://doi.org/10.1002/jcc.22885
Perdew JP, Burke K, Wang Y (1996) Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys Rev B 54(23):16533–16539
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–5652. https://doi.org/10.1063/1.464913
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110(13):6158–6170. https://doi.org/10.1063/1.478522
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393(1):51–57. https://doi.org/10.1016/j.cplett.2004.06.011
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys Chem Chem Phys 10(44):6615–6620. https://doi.org/10.1039/B810189B
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865–3868
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J Chem Phys 72(10):5639–5648. https://doi.org/10.1063/1.438980
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80(7):3265–3269. https://doi.org/10.1063/1.447079
Cancès E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 107(8):3032–3041. https://doi.org/10.1063/1.474659
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88(6):899–926. https://doi.org/10.1021/cr00088a005
Kar S, Roy J, Leszczynska D, Leszczynski J (2017) Power conversion efficiency of arylamine organic dyes for dye-sensitized solar cells (DSSCs) explicit to cobalt electrolyte: understanding the structural attributes using a direct QSPR approach. Comput Des 5(1):2
Kar S, Roy JK, Leszczynski J (2017) In silico designing of power conversion efficient organic lead dyes for solar cells using todays innovative approaches to assure renewable energy for future. NPJ Computational Materials 3(1):22. https://doi.org/10.1038/s41524-017-0025-z
Guerra M (1999) Role of standard diffuse functions for computing hyperfine splitting constants in radical anions. J Phys Chem A 103(30):5983–5988. https://doi.org/10.1021/jp990012a
Ranck JP, Johansen H (1972) Polarization functions and geometry optimization in ab initio calculations of the rotational barrier in hydrogen peroxide. Theor Chim Acta 24(4):334–345. https://doi.org/10.1007/bf01007551
Qu Z-W, Zhu H, May V, Schinke R (2009) Time-dependent density functional theory study of the electronic excitation spectra of chlorophyllide a and pheophorbide a in solvents. J Phys Chem B 113(14):4817–4825. https://doi.org/10.1021/jp805804r
Aziz SG, Elroby SAK, Hilal RH, Osman OI (2014) Theoretical and computational studies of conformation, natural bond orbital and nonlinear optical properties of cis-N-phenylbenzohydroxamic acid. Computational and Theoretical Chemistry 1028:65–71. https://doi.org/10.1016/j.comptc.2013.12.001
Skoog DA, Holler FJ, Crouch SR (2007) Principles of instrumental analysis. Brooks/Cole. Thomson Learning, Melbourne
Casanova D (2011) The role of the π linker in donor–π–acceptor organic dyes for high-performance sensitized solar cells. ChemPhysChem 12(16):2979–2988. https://doi.org/10.1002/cphc.201100520
Foster JP, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102(24):7211–7218. https://doi.org/10.1021/ja00544a007
Reed AE, Weinhold F (1983) Natural bond orbital analysis of near-Hartree–Fock water dimer. J Chem Phys 78(6):4066–4073. https://doi.org/10.1063/1.445134
Reed AE, Weinhold F (1985) Natural localized molecular orbitals. J Chem Phys 83(4):1736–1740. https://doi.org/10.1063/1.449360
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83(2):735–746. https://doi.org/10.1063/1.449486
Hrobárik P, Sigmundová I, Zahradník P, Kasák P, Arion V, Franz E, Clays K (2010) Molecular engineering of benzothiazolium salts with large quadratic hyperpolarizabilities: can auxiliary electron-withdrawing groups enhance nonlinear optical responses? J Phys Chem C 114(50):22289–22302. https://doi.org/10.1021/jp108623d
Asiri AM, Khan SA, Al-Amoudi MS, Alamry KA (2012) Synthesis, characterization, absorbance, fluorescence and non linear optical properties of some donor acceptor chromophores. Bull Kor Chem Soc 33(6):1900–1906. https://doi.org/10.5012/bkcs.2012.33.6.1900
Kaatz P, Donley EA, Shelton DP (1998) A comparison of molecular hyperpolarizabilities from gas and liquid phase measurements. J Chem Phys 108(3):849–856. https://doi.org/10.1063/1.475448
Osman OI (2017) DFT study of the structure, reactivity, natural bond orbital and hyperpolarizability of thiazole azo dyes. Int J Mol Sci 18(2):239. https://doi.org/10.3390/ijms18020239
Bures F (2014) Fundamental aspects of property tuning in push-pull molecules. RSC Adv 4(102):58826–58851. https://doi.org/10.1039/C4RA11264D
Garza AJ, Osman OI, Asiri AM, Scuseria GE (2015) Can gap tuning schemes of long-range corrected hybrid functionals improve the description of hyperpolarizabilities? J Phys Chem B 119(3):1202–1212. https://doi.org/10.1021/jp507226v
Cardoso C, Abreu PE, Milne BF, Nogueira F (2010) Computational study of molecules with high intrinsic Hyperpolarizabilities. J Phys Chem A 114(39):10676–10683. https://doi.org/10.1021/jp105707q
Acknowledgements
This project was funded by Deanship of Scientific Research (DSR) of King Abdulaziz University, Jeddah under grant no. G-178-130-1437. The authors, therfore, acknowledge with thanks DSR support for Scientific Research. The computational work described in this paper was supported by King Abdulaziz University High Performance Computing Center (AZIZ supercomputer, http://hpc.kau.edu.sa).
Funding
Deanship of Scientific Research (DSR) and King Abdulaziz University, Jeddah [grant no. G-178-130-1437].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The manuscript has not been previously published, is not currently submitted for review to any other journal, and will not be submitted elsewhere before a decision is made by this journal.
Rights and permissions
About this article
Cite this article
Aziz, S.G., Osman, O.I., Elroby, S.A. et al. Proton-coupled electron transfer in dye-sensitized solar cells: a theoretical perspective. Struct Chem 29, 983–997 (2018). https://doi.org/10.1007/s11224-018-1080-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1080-x