Abstract
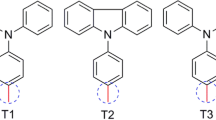
In this study, we are interested to the effect of the variation in donor groups (D) mainly carbazole, triphenylamine, diethylaniline and phenothiazine, on a series of organic compounds, using Density-Functional Theory (DFT) and Time-Dependent Becke–Half and Half–Lee–Yang–Parr's (TD-BHandHLYP). The aim is to elucidate the different geometrical and optoelectronic properties, as well as the charge transfer parameters (Ionization potential (IP), Electron affinity (EA), Reorganization energy (λ), Light harvesting efficiency (LHE), Open circuit voltage (Voc), Injection energy (ΔGinject) and Regeneration energy (ΔGreg)), chemical reactivity parameters (Electronegativity (χ), Chemical potential (μ), and Electrophilicity index (ω)) of the studied dyes and intermolecular interactions dyes/semiconductor. Using triphenylamine and diethylaniline as donors (D), we have been able to get the lowest Egap energies, with respective values roughly equivalent to 1.77 and 1.78 eV and high maximum absorption wavelengths (607.84 and 609.70 nm) when compared to the other donor groups. Likewise, the introduction of these units increased the photon-current conversion capacity, characterized by better LHE values (0.980 and 0.975 eV, respectively) and higher Voc (0.91 and 0.95 eV, respectively), which would improve the charge transfer performance and chemical reactivity indices.








Similar content being viewed by others
Data availability
All data are available and mainly the Cartesian Coordinates of all the studied dyes.
References
M. Grätzel, Acc Chem Res. (2009). https://doi.org/10.1021/ar900141y
M. Grätzel, J. Photochem. Photobiol C Photochem Rev. (2003). https://doi.org/10.1016/S1389-5567(03)00026-1
Y. Li, J. Liu, D. Liu, X. Li, Y. Xu, Comput Mater Sci. (2019). https://doi.org/10.1016/j.commatsci.2019.01.033
M. Megala, B.J.M. Rajkumar, J Comput Electron. (2018). https://doi.org/10.1007/s10825-018-1195-8
B. Nagarajan, C.D. Athrey, R. Elumalai, S. Chandran, D. Raghavachari, Photochem Photobiol Chem. (2021). https://doi.org/10.1016/j.jphotochem.2020.112820
A.R. Marri, H. Flint, E.A. Gibson, J. Fielden, Dyes and Pig. (2022). https://doi.org/10.1016/j.dyepig.2022.110244
M. Lazrak, H. Toufik, S.M. Bouzzine, H. Bih, F. Lamchouri, RHAZES: G and App Chem. 2, 8 (2018)
P. Gnida, A. Slodek, P. Chulkin, M. Vasylieva, A.K. Pająk, A. Seweryn, M. Godlewski, B.S. Witkowski, G. Gorol, E.S. Balcerzak, Dyes and Pig. (2022). https://doi.org/10.1016/j.dyepig.2022.110166
Z.-D. Sun, M. He, K. Chaitanya, X.-H. Ju, Mater. Chem. Phys. (2020). https://doi.org/10.1016/j.matchemphys.2020.122943
A. Arunkumar, S. Shanavas, P.M. Anbarasan, J Comput Electron. (2018). https://doi.org/10.1007/s10825-018-1226-5
J. Burschka, N. Pellet, S.J. Moon, R.H. Baker, P. Gao, M.K. Nazeeruddin, M Gra¨tzel. Nature (2013). https://doi.org/10.1038/nature12340
M. Liu, M.B. Johnston, H.J. Snaith, Nature (2013). https://doi.org/10.1038/nature12509
S.E. Mzioui, S.M. Bouzzine, İ Sidir, M. Bouachrine, M.N. Bennani, M. Bourass, M. Hamidi, J Mol Model. (2019). https://doi.org/10.1007/s00894-019-3963-1
H. Zhang, Z.E. Chen, J. Hu, Y. Hong, Dyes Pig. (2019). https://doi.org/10.1016/j.dyepig.2019.01.033
D.M. Almenningen, H.E. Hansen, M.F. Vold, A.F. Buene, V. Venkatraman, S. Sunde, B.H. Hoff, O.R. Gautum, Dyes Pig (2021). https://doi.org/10.1016/j.dyepig.2020.108951
R. Royo, A.D. Celorrio, S. Franco, R. Andreu, J. Orduna, Dyes and Pig. (2022). https://doi.org/10.1016/j.dypepig.2022.110566
I. Duerto, S. Sarasa, D. Barrios, Jesús Orduna, B Villacampa, M J Blesa. Dyes Pigm. (2022). https://doi.org/10.1016/j.dyepig.2022.110310
B. Xu, E. Sheibani, P. Liu, J. Zhang, H. Tian, N. Vlachopoulos, G. Boschloo, L. Kloo, A. Hagfeldt, L. Sun, Adv Mater. (2014). https://doi.org/10.1002/adma.201402415
P.J. Pacheco-Li, A. Navarro, J. Tolosa, M.́ oMoral, C. Martín, I.́ Bravo, J. Hofkens, J.C. García-Martínez, A G on-Ruiz. Dyes and Pig. (2022). https://doi.org/10.1016/j.dyepig.2022.110105
C. Chen, J.Y. Liao, Z. Chi, B. Xu, X. Zhang, D.B. Kuang, Y. Zhang, S. Liu, Jiarui Xu. J Mater Chem. (2012). https://doi.org/10.1039/c2jm30254c
K. Hara, T. Sato, R. Katoh, A. Furube, T. Yoshihara, M. Murai, M. Kurashige, S. Ito, A. Shinpo, S. Suga, H. Arakawa, Adv Funct Mater. (2005). https://doi.org/10.1002/adfm.200400272
M. Liang, J. Chen, Chem Soc Rev. (2013). https://doi.org/10.1039/c3cs35372a
G. Dyrda, R. Słota, M.A. Broda, G. Mele, Res Chem Intermed. (2016). https://doi.org/10.1007/s11164-015-2245-5
F. Zasada, J. Janas, W. Piskorz, Z. Sojka, Res. Chem. Intermed. (2017). https://doi.org/10.1007/s11164-016-2798-y
S. Schinzel, M. Bindl, M. Visseaux, H. Chermette, J. Phys. Chem. A. (2006). https://doi.org/10.1021/jp060876d
M. Grätzel, Nature 414(6861), 338–344 (2001)
A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo, H. Pettersson, Chem. Rev. (2010). https://doi.org/10.1021/cr900356p
M.J. Frisch, G.W. Trucks, B. Schlegelh, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Peterson, Gaussian IncWallingford CT 121, 150 (2009)
C. Negri, E. Borfecchia, A. Martini, G. Deplano, K.A. Lomachenko, T.V.W. Janssens, G. Berlier, S. Bordiga, Res Chem Intermed (2021). https://doi.org/10.1007/s11164-020-04350-1
Z. Xu, Y. Li, W. Zhang, S. Yuan, L. Hao, T. Xu, X. Lu, Spectrochim Acta A Mol Biomol Spectrosc. (2019). https://doi.org/10.1016/j.saa.2019.01.002
R.H. Hertwig, W. Koch, Chem. Phys. Lett. (1997). https://doi.org/10.1016/S0009-2614(97)00207-8
I. Althagafi, N.E. Metwaly, Arab. J. Chem. (2021). https://doi.org/10.1016/j.arabjc.2021.103080
K.L. Schuchardt, B.T. Didier, T. Elsethagen, L. Sun, V. Gurumoorthi, J. Chase, J. Li, T.L. Windus, J Chem Inf Model 47(3), 1045 (2007)
B.P. Pritchard, D. Altarawy, B. Didier, T.D. Gibson, TLWindus. J Chem Inf Model 59(11), 4814 (2019)
J.P. Perdew, R.G. Parr, M. Levy, J.L. Balduz, Phys Rev Let. (1982). https://doi.org/10.1103/PhysRevLett.49.1691
M. Lazrak, H. Toufik, S.M. Bouzzine, F. Lamchouri, Res. Chem. Intermed. (2020). https://doi.org/10.1007/s11164-020-04184-x
I.M. Walton, J.M. Cox, C.A. Benson, D.G. Patel, Y.S. Chen, J.B. Benedict, New J Chem. (2016). https://doi.org/10.1039/C5NJ01718A
N.A. Wazzan, J Comput Electron. (2019). https://doi.org/10.1007/s10825-019-01308-4
E. Hosseinzadeh, N.L. Hadipour, G. Parsafar, J Photochem Photobiol Chem. (2017). https://doi.org/10.1016/j.jphotochem.2016.10.010
A.D. Becke, J. Chem. Phys. (1993). https://doi.org/10.1063/1.464304
Z.M.E. Fahim, S.M. Bouzzine, A.A. Youssef, M. Bouachrine, M. Hamidi, Comput Theor Chem. (2018). https://doi.org/10.1016/j.comptc.2018.01.002
S.B. Novir, S.M. Hashemianzadeh, Spectrochim Acta A Mol Biomol Spectrosc. (2015). https://doi.org/10.1016/j.saa.2015.02.026
M. Lazrak, H. Toufik, S. Ennehary, S.M. Bouzzine, F. Lamchouri, Orbital Elec J Chem. (2022). https://doi.org/10.17807/orbital.v14i1.1682
M. Lakshmanakumar, S. Sriram, D. Balamurugan, J. Compu, Electron. (2018). https://doi.org/10.1007/s10825-018-1189-6
A.N. Ossai, S.C. Ezike, P. Timtere, A.D. Ahmed, Chem Phys Impact. (2021). https://doi.org/10.1016/j.chphi.2021.100024
A. Tripathi, A. Ganjoo, P Chetti (2020). https://doi.org/10.1016/j.solener.2020.08.084
A.R. Obasuyi, D.G. Mitnik, N.F. Holguín, J Comput Electron. (2019). https://doi.org/10.1007/s10825-019-01331-5
S. Mandal, G.R. Kandregula, K. Ramanujam, J Photochem Photobiol Chem. (2020). https://doi.org/10.1016/j.jphotochem.2020.112862
R. Katoh, A. Furube, T. Yoshihara, K. Hara, G. Fujihashi, S. Takano, S. Murata, H. Arakawa, M. Tachiya, J. Phys Chem B. (2004). https://doi.org/10.1021/jp031260g
C.R. Zhang, L. Liu, J.W. Zhe, N.Z. Jin, Y. Ma, L.H. Yuan, M.L. Zhang, Y.Z. Wu, Z.J. Liu, H S Chen Int J Mol Sci. (2013). https://doi.org/10.3390/ijms14035461
A.B. Lovins, Energy Env Sci. (2009). https://doi.org/10.1039/B814525N
L.J. He, J. Chen, F.Q. Bai, R. Jia, J. Wang, H.-X. Zhang, Dyes Pig. (2017). https://doi.org/10.1016/j.dyepig.2017.02.023
H. Toufik, S.M. Bouzzine, O. Ninis, F. Lamchouri, M. Aberkane, M. Hamidi, M. Bouachrine, Res. Chem. Intermed. (2012). https://doi.org/10.1007/s11164-011-0469-6
J. Xu, L. Zhu, D. Fang, B. Chen, L. Liu, L. Wang, W. Xu, Chem Phys Chem. (2012). https://doi.org/10.1002/cphc.201200273
L. Han, Y. Ke, X. Liu, S. Jiang, J Mol Struct. (2019). https://doi.org/10.1016/j.molstruc.2019.02.008
G. Deogratias, O.S. Al-Qurashi, N. Wazzan, N. Seriani, T. Pogrebnaya, A Pogrebnoi Struct Chem. (2020). https://doi.org/10.1007/s11224-020-01596-8
R.M. El-Shishtawy, A.M. Asiri, S.G. Aziz, S.A.K. Elroby, J Mol Model. (2014). https://doi.org/10.1007/s00894-014-2241-5
S. Ennehary, H. Toufik, S.M. Bouzzine, F. Lamchouri, J. Comput. Electron. (2020). https://doi.org/10.1007/s10825-020-01486-6
M. Grätzel, Nature 414, 7 (2021)
V.T.T. Huong, T.B. Tai, M.T. Nguyen, J Phys Chem A. (2014). https://doi.org/10.1021/jp500899k
P.J. Hay, W.R. Wadt, J Chem Phys. (1985). https://doi.org/10.1063/1.448799
N. Santhanamoorthi, C.M. Lo, J.C. Jiang, J Phys Chem Lett. (2013). https://doi.org/10.1021/jz302101j
P. Guo, R. Ma, L. Guo, L. Yang, J. Liu, X. Zhanga, X. Pan, S. Daib, J Mol Graph Model. (2010). https://doi.org/10.1016/j.jmgm.2010.10.002
P. Pounraj, V. Mohankumar, M.S. Pandian, P. Ramasamy, H H India. (2019). https://doi.org/10.1063/1.5113184
A.B.E. Meligy, N. Koga, S. Iuchi, K. Yoshida, K. Hirao, A.H. Mangood, A.M.E. Nahas, J. Photochem. Photobiol. Chem. (2018). https://doi.org/10.1016/j.jphotochem.2018.08.036
L.L. Estrella, M.P. Balanay, D.H. Kim, J Phys Chem. A. (2016). https://doi.org/10.1021/acs.jpca.6b03271
A. Mahmood, S.U.D. Khan, U.A. Rana, J Comput Electron. (2014). https://doi.org/10.1007/s10825-014-0628-2
V.A. Chiykowski, B. Lam, C. Du, C.P. Berlinguette, Chem Commun. (2017). https://doi.org/10.1039/C6CC09178D
M. Xu, M. Zhang, M. Pastore, R. Li, F. De Angelis, P. Wang, Chem Sci. (2012). https://doi.org/10.1039/c2sc00973k
M.M. Jadhav, T.H. Chowdhury, I. Bedja, D. Patil, A. Islam, N. Sekar, Dyes Pig. (2019). https://doi.org/10.1016/j.dyepig.2019.02.045
M.M. Raikwar, D.S. Patil, E. Mathew, M. Varghese, I.H. Joe, N. Sekar, J Photochem Photobiol Chem. (2019). https://doi.org/10.1016/j.jphotochem.2018.12.035
N.S.A. Fahdan, A.M. Asiri, A. Irfan, S.A. Basaif, R.M.E. Shishtawy, J Mol Model. (2014). https://doi.org/10.1007/s00894-014-2517-9
G. Deogratias, N. Seriani, T. Pogrebnaya, A. Pogrebnoi, J. Mol. Graph. Model. (2020). https://doi.org/10.1016/j.jmgm.2019.107480
H. Tian, X. Yang, J. Pan, R. Chen, M. Liu, Q. Zhang, A. Hagfedtl, L. Sun, Adv Funct Mater. (2008). https://doi.org/10.1002/adfm.200800516
W. Sang-aroon, S. Saekow, V. Amornkitbamrung, J Photochem Photobiol Chem. (2012). https://doi.org/10.1016/j.jphotochem.2012.03.014
S. Ennehary, H. Toufik, M. Lazrak, S.M. Bouzzine, F. Lamchouri, J Mol Model. (2021). https://doi.org/10.1007/s00894-021-04733-0
J. Burschka, A. Dualeh, F. Kessler, E. Baranoff, N.C. Ha, C. Yi, M.K. Nazeeruddin, M. Grätzel, J. Am. Chem. Soc. (2011). https://doi.org/10.1021/ja207367t
J.M. Juma, S.A. Vuai, J Chem Res. (2021). https://doi.org/10.1177/1747519821994518
R.S. Rojo, J.B. López, D.G. Mitnik, Phys. Chem. Chem. Phys. (2015). https://doi.org/10.1039/C5CP01387A
P.K. Chattaraj, B. Maiti, J. Am. Chem. Soc. (2003). https://doi.org/10.1021/ja0276063
R.G. Pearson, J Chem Sci. (2005). https://doi.org/10.1007/BF02708340
S. Liu, J Chem Sci. (2005). https://doi.org/10.1007/BF02708352
R.G. Parr, R.G. Pearson, J. Am. Chem. Soc. (1983). https://doi.org/10.1021/ja00364a005
O. Osman, Int J Mol Sci. (2017). https://doi.org/10.3390/ijms18020239
B. Regan, M. Grätzel, Nature 353, 737 (1991)
Y. Wang, D.J. Doren, Sol. State Com. (2005). https://doi.org/10.1016/j.ssc.2005.05.042
M.B. Manaa, N. Issaoui, N. Bouaziz, A.B. Lamine, J. of Materials Res. and Tech (2020). https://doi.org/10.1016/j.jmrt.2019.11.045
A. Irfan, Computat. and Theore. Chem. (2019). https://doi.org/10.1016/j.comptc.2019.04.008
J.K. Roy, S. Kar, J. Leszczynski, Sci. Rep. (2018). https://doi.org/10.1038/s41598-018-29368-9
M. Pastore, F. De Angelis, ACS Nano (2009). https://doi.org/10.1021/nn901518s
P. Chen, J.H. Yum, F. De Angelis, E. Mosconi, S. Fantacci, S.J. Moon, R.H. Baker, J. Ko, M.K. Nazeeruddin, M. Gratzel, Nano Lett. (2009). https://doi.org/10.1021/nl901246g
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SE did most of the practical work as part of a PhD thesis supervised by HT and prepared the manuscript. HT designed and coordinated the study, participated in article preparation, corrected the manuscript and edited the final version and submitted it for publication. SiMB participated in study designed, helped to improve the manuscript and critically revised the manuscript. ML contributed to data analysis. FL participated in study designed, helped to improve the manuscript and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethics approval and consent to participate Compliance with ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ennehary, S., Toufik, H., Bouzzine, S.M. et al. Theoretical investigation for dye-sensitized solar cells: effect of donor variation on the optoelectronic properties and charge transfer parameters. Res Chem Intermed 49, 1731–1754 (2023). https://doi.org/10.1007/s11164-023-04971-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-04971-2