Abstract
This article presents the syntheses, crystal structures, topological features and magnetic properties of two NiII/NaI coordination clusters formulated [Ni II3 Na(L1)3(HL1)(MeOH)2] (1) and [Ni II6 Na(L1)5(CO3)(MeO)(MeOH)3(H2O)3]·4(MeOH) 2(H2O) [2 4(MeOH) 2(H2O)] where H2L1 is the semi-rigid Schiff base ligand (E)-2-(2-hydroxy-3-methoxybenzylideneamino)-phenol). Compound 1 possesses a rare Ni II3 NaI cubane (3M4-1) topology, and compound 2 is the first example in polynuclear Ni/Na chemistry that exhibits a 2,3,4M7-1 topology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and review
One of the most complex categories of coordination compounds are polynuclear coordination clusters (CCs) that incorporate multiple metal ions into a single molecular entity and are linked by bridging ligands [1]. These entities are of great interest for their aesthetically beautiful structures [2–4], unexpected transformations [5–7] and potential applications in magnetism [8–10], luminescence [11–15], catalysis [16–18], etc. Paramagnetic transition metal CCs are of intense interest and have attracted a vast amount of attention since the discovery that some CCs behave as single-molecule magnets (SMMs) [19–21]. The NiII (d8) ion has second-order orbital angular momentum, and zero-field splitting (ZFS) which can result in significant single-ion anisotropy and potentially in molecules exhibiting interesting magnetic properties [22, 23]. The interest in polynuclear Ni(II) coordination chemistry was first captured when the first Ni(II)-based SMM, a Ni12 complex, was reported in 2001 by Cadiou et al. [24]. Ever since, there have been a number of homometallic polynuclear NiII CCs with high nuclearities including, Ni5 [7, 25], Ni6 [26], Ni7 [27, 28], Ni8 [29–32], Ni9 [33, 34], Ni11 [35], Ni12 [7], Ni13 [36], Ni14 [37], Ni20 [38], Ni21 [39], Ni24 [40] and Ni26 [41], and many of these display interesting magnetic properties including ferromagnetic, ferrimagnetic coupling, diamagnetism and SMM behaviour.
Clusters of this size incorporate simple modified ligands with a wide variety of coordination modes for bridging, such as diethanolamine [42], Schiff base [43], carbide [44, 45] and carboxylate [46]. The introduction of bridging groups can increase the nuclearity of a CC. Carbonate anions offer a diverse range of bridging modes within cluster type molecules. A number of high-nuclearity CCs have been based on carbonate moieties [47, 48]. While Ni(II) CCs with bridging carbonate ligands are known, structural factors and magnetic exchange within these clusters greatly vary due to the large number of coordination modes of the CO3 2− anion [39, 49, 50]. Some interesting examples include, a Ni6 containing a carbonato bridge [51] and an Ni12 where four Ni4O4 units are templated around a central CO3 2− anion core [52]. On the other hand, the first reported mixed NiII/NaII CC was reported in 1976 by Jonas for potential small-molecule activation [53]. Since then, a number of NiII/NaII CCs have been reported, targeting for high-nuclearity clusters and interesting magnetic properties: Ni4Na2 [54], Ni4Na5 [55], Ni4Na3 [56], Ni4Na4 [57, 58] and others [59–62]. However, since 2007, NiII/NaI CCs with nuclearity over 10 have been reported far more frequently. The highest reported of these is a Ni18Na6 cluster [63] and the second Ni16Na4 [64], both synthesised by calix [4] arene-type ligands and the third is a Ni16Na2 cluster. In addition, two Ni12Na2 clusters were reported by Christou et al. [37, 65]. In all cases, similar anti-ferromagnetic behaviour was observed.
The diprotic Schiff base ligand (E)-2-(2-hydroxy-3-methoxybenzylideneamino)-phenol (H2L1, Scheme 1) initially reported in 1971 to capturing UO2 [66] can be synthesised in almost quantitative yields [67] and has two pockets that can coordinate to metal centres. Previously, this ligand has been involved in the synthesis of homometallic [68–71] and heterometallic CCs [72–75]. We recently employed this ligand in 3d/4f chemistry to synthesise a family of homogeneous efficient catalysts towards a domino reaction [76]. Interestingly, when H2 L1 was employed in Ni(II) chemistry, a tetranuclear Ni4 CC exhibiting ferromagnetic interactions at low temperatures was isolated. [71] With the interest of introducing carbonate anions into a system, there are three key methods: direct addition of carbonate or bicarbonate [14], atmospheric fixation of carbon dioxide [77] and in situ decomposition of ligands [78].
Having all these in mind, in this article, we study the influence of the presence of Na cations and CO3 2− anions on the nuclearity of the given chemical system Ni(II)/H2L1 and we report two compounds formulated [Ni II3 Na(L1)3(HL1)(MeOH)2] (1) and [Ni II6 NaI(L1)5(CO3)(MeO)(MeOH)3(H2O)3]·4(MeOH) 2(H2O) [2 4(MeOH) 2(H2O)]. Topological aspects and magnetic properties of these compounds are further discussed.
Experimental
Materials
Chemicals (reagent grade) were purchased from Sigma-Aldrich and Alfa Aesar. All experiments were performed under aerobic conditions using materials and solvents as received.
Instrumentation
IR spectra of the samples were recorded over the range of 4000–650 cm−1 on a Perkin-Elmer Spectrum One FT-IR spectrometer fitted with a UATR polarization accessory.
X-ray diffraction
Crystallography
Data for 1 and 2·4(MeOH) were collected at the National Crystallography Service, University of Southampton [79] using a Rigaku Saturn 724+ area detector mounted at the window of an FR-E+ rotating anode generator with a Mo anode (λ = 0.71075 Å) under a flow of nitrogen gas at 150(2) K for 1 and 100(2) K for 2·4(MeOH). Both structures were determined using Olex2 [80], solved using either Superflip [81] or SHELXT [82, 83] and refined with SHELXL-2014 [84]. All non-H atoms were refined with anisotropic thermal parameters, and H atoms were introduced at calculated positions and allowed to ride on their carrier atoms. For 1, attempts to model the lattice solvents (2MeOH) were unsuccessful despite multiple data collections. Therefore, the solvent mask function of Olex2 [80] was employed to remove the contribution of the electron density associated with those molecules from the intensity data. Geometric/crystallographic calculations for all structures were performed using PLATON [85], Olex2 [80] and WINGX [82] packages; graphics were prepared with Crystal Maker [86] CCDC 1476870-1476871 (Tables 1, 2, 3).
Magnetic studies
Magnetic susceptibility measurements were carried out on polycrystalline samples with a MPMS5 Quantum Design susceptometer working in the range 30–300 K under external magnetic field of 0.3 T and under a field of 0.03 T in the 30–2 K range to avoid saturation effects. Diamagnetic corrections were estimated from Pascal tables. The quality of the fitting is parameterised as the factor R = (χ M T exp−χ M T calc)2/(χ M T exp)2.
Synthesis of H2L1
o-Vanillin (0.025 mol, 3.35 g) and 2-amino-phenol (0.025 mol, 2.73 g) were dissolved in MeOH (5 mL). The suspension was refluxed for 1 h, during which time a bright orange solid precipitated. After cooling to room temperature, the solid was filtered off and washed with cold MeOH and Et2O. The solid was dried in vacuo. Yield 99 %. 1H NMR (500 MHz, DMSO-d6) δ 9.75–9.71 (m, 1H), 8.95 (s, 1H), 7.36 (dd, J = 8.0, 1.6 Hz, 1H), 7.21–6.99 (m, 3H), 6.96 (dd, J = 8.1, 1.4 Hz, 1H), 6.91–6.81 (m, 2H), 3.80 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 162.01, 152.27, 151.42, 148.63, 134.95, 128.46, 124.26, 120.05, 119.96, 119.70, 118.37, 116.97, 115.71, 56.34, 40.62, 40.53, 40.45, 40.36, 40.28, 40.19, 40.11, 40.03, 39.95, 39.86, 39.69, 39.53.
Synthesis of 1
Ni(ClO4)2.6H2O (0.1 mmol, 37 mg), H2 L1 (0.1 mmol, 24 mg) and Na2CO3 (0.1 mmol, 10 mg) were suspended in MeOH (20 mL) and stirred for 1 h. The solution was filtered and the filtrate left for slow evaporation. After 14 days, small brown crystals of 1 were collected and washed with Et2O. [Yield = 45 % calculated based on Ni(II)]. IR (ν, cm−1) = 3402, 3291, 1780, 1609, 1552, 1454, 1388, 1291, 1224, 1182, 1073, 1033, 964, 820, 733, 635. CHN [Ni3Na(C14H11NO3)3(C14H12NO3)(CH3OH)2]; observed C-56.63 %; H-4.47 %, N-4.43 % (expected); C-56.76 %; H-4.35 %; N-4.56 %.
Synthesis of [2 4(MeOH) 2(H2O)]
A similar synthetic procedure to 1 was followed; however, after filtration, the solution was placed in a vial which in turn was placed in a larger vial that contained a saturated aqueous solution of Na2CO3. A few drops of concentrated HCl were added to the saturated solution, and the vial was immediately sealed. After 5 days, large brown block-like crystals of compound [2 4(MeOH)] were collected. (Yield = 60 % calculated based on Ni(II)). IR (ν, cm−1) = 3288, 1604, 1552, 1451, 1388, 1294, 1229, 1182, 1075, 1037, 966, 818, 736, 637. CHN [Ni6Na(C14H11NO3)5(CO3)(CH3O)(CH3OH)3(H2O)3] (observed); C-49.51 %; H-4.11 %; N-3.79 %; (expected) C-49.52 %; H-4.21 %; N-3.85 %.
Results and discussion
Two NiII/NaII CCs were synthesised. The aim to use Na2CO3 as the base to introduce carbonate bridges to the system results in the presence of one NaI in both CCs, while both compounds have different structures to the previously reported homometallic NiII CC with H2L1 [69]. In 1, the use of Na2CO3 did not result in the introduction of carbonate bridging groups; however, in 2, where the reaction solution was exposed to a high CO2 atmosphere during slow evaporation, a single CO3 2− anion is observed. 1 and 2 are new additions to a large family of previously reported NiII/NaI CCs.
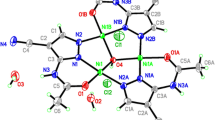
1 crystallises in the monoclinic space group P21/n and contains one molecule in the asymmetric unit cell. Compound 1 contains three NiII ions, one NaI ion, three doubly deprotonated (L1) ligands, one mono deprotonated (HL1 −) ligand and three coordinated CH3OH solvent. Each NiII ion coordinates to six heteroatoms and displays a distorted octahedral geometry. There are three observed coordination modes for the doubly deprotonated ligands (L1) (Scheme 2); in each, a NiII ion occupies the ONO pocket between the phenoxido and imido atoms. In the singly protonated (HL1) ligand coordination mode (Scheme 2) [69, 71–74, 87–91], a NiII ion is observed occupying the OO pocket between the methoxido and phenoxide ligand atoms. Interestingly, L1 demonstrates a twisted out-of-plane geometry between the two aromatic rings with a torsion angle of −100.679° along the C = C-N = C bond, whilst the L1 Ligand aromatic rings are close to being in-plane with torsion angles between −166.840° and 174.23°. In addition to this, both NiII and the NaI are observed bridging from the phenoxido atoms. The NaI ion displays a distorted octahedral geometry with average Na–O bond distances ranging from 2.272 to 2.648A. Then, three NiII ions all display distorted octahedral geometry with average Ni–O bind distances ranging from 1.981 to 2.343A. Four μ3-Ophenoxo donors are placed in the corners of the cube linking the four cations. Ni–O–Ni bridges form three faces of the cube with bond angles of 97.0(1)°/97.7(1)°, 97.18(9)°/99.87(9)° and 93.38(9)°/102.7(1)°.
According to a nomenclature, developed by some of us [92], the decorated core of this compound is assigned 3M4-1 (Fig. 1). An extended literature survey reveals only three previously reported complexes with Ni3Na core topologies, each of which are synthesised from similar Schiff base ligands and coordination pockets to H2 L1 [93, 94]. The most recently reported of these Zhang et al. [93] shares the same 3M4-1 topology as 1, making 1 the second example of a Ni II3 /NaI cubane.
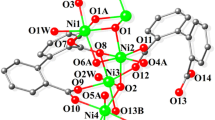
Compound 2 crystallises in the triclinic P-1 space group and contains two molecules in the asymmetric unit. The molecule of 2 contains six NiII ions, one NaI ion, five doubly deprotonated L1 ligands, one bridging (µ5-CO3), one deprotonated MeOH solvent molecule with three CH3OH and three H2O molecules which fulfil the coordination geometry of the metal ions.
All six NiII ions have a distorted octahedral geometry. The L1 demonstrates four different bridging modes (Scheme 2) with NiII ions observed, occupying the (ONO) and (OO) pocket as well as bridging from the phenoxido, methoxido and imine groups. The NaII ion is seven-coordinated, and the coordination geometry can best be described as a capped trigonal prism determined by SHAPE software. The NaI ion coordinates to the (OO) pocket between the methoxide and phenoxido atoms of three ligands with the seventh position fulfilled by a H2O molecule. The CO3 2− entity bridges Ni2, Ni4, Ni5 and Ni6 through a (μ5-CO3) ion. Na–O bond distances range from 2.282 to 2.625A. The Ni–O and Ni–N distances vary between 1.983(6) and 2.321(4) Å, while the Ni–O–Ni angles mediated by the Ophenoxo donors vary from 88.88(17) and 101.8(2)°, those mediated by the μ3-OMe bridge, that links Ni1, Ni2 and Ni3, exhibit bond angles ranging between 95.74(19) and 100.2(2)° and Ni5-O22-Ni6, mediated by one Ocarbonate atom reach 130.4(2)°.
Adopting our topological approach, the core of compound 2 can be enumerated as 2,3,4M7-1 [92]. The 2,3,4M7-1 core topology can be viewed as two lozenges fused with a shared apex vertices (Fig. 2 lower); this can be otherwise described as a “butterfly” motif, where the shared vertices are the body and the protruding lozenges the “wings”. The first reported compound of this topology was a mixed valence MnII/MnIII complex in 1991 [95]. This was subsequently followed by a number of homometallic CCs, including MnIII [96] and PbII [97]. The first heterometallic complex of this topology was reported in 2012 with the core topology of Zn5Na2 [98] with the ((2,3-dihydroxypropylamino)methyl)phenol ligand (Scheme 3 H3L5). The metal ion positions of this core topology differ to the reported compound 2. The NaI ions occupy the two apex vertices of the two fused lozenges with all other positions occupied by the 3d ZnII ions. In both examples, the fused vertice is occupied by a 3d ion (NiII/ZnII). Metal ion geometries widely differ in both complexes, with NaI ions possessing severely distorted octahedral geometry and ZnII ions tetrahedral. Both of these complexes are formed from Schiff base ligands incorporating the 2-hydroxy-benzaldehyde moiety, which provides a similar coordination environment, with further bridging attributed to the third hydroxyl group in H3L5. To the best of our knowledge, 2 is the first example of an NiII/NaI CC exhibiting the 2,3,4M7-1 topology. Moreover, ignoring the existence of the Na ion, the motif of the hexanuclear Ni6 unit can be enumerated as 1,2,2,3,3M6-1 (Figure S3), which has been reported only once before in Zinc chemistry [99].
Magnetic studies
The χ MT value at room temperature for compound 1 is 3.97 cm3 Kmol−1, increase in cooling to a maximum value of 4.50 cm3 Kmol−1 at 13 K, prior to decrease at low temperature, indicating a dominant weak ferromagnetic interaction. Fit of the experimental data was performed with PHI [100] program and applying the Hamiltonian:
including a D ion parameter. Considering the D ion parameter, an excellent fit of the experimental data was obtained for the parameters J 1 = 3.6 cm−1, J 2 = −2.3 cm−1, D = 1.2 cm−1, g = 2.26 and R = 4.2·10−5 but this was not the case when the isotropic Hamiltonian were applied. The shape of the χ MT plot corresponds to a weak ferromagnetic coupling with a strong decrease at low temperature due to combination of the AF component and the ZFS of the same order of magnitude than J.
The χ MT value at room temperature for 2 is 7.1 cm3 Kmol−1 at room temperature and on cooling increases slight up to a rounded maximum of 7.34 cm3 Kmol−1 at 45 K. Below this temperature, the χ MT value decreases down to 5.94 cm3 Kmol−1 at 2 K. From the structural data, compound 2 consists of a Ni4 butterfly subunit linked by means of different kinds of bridges to two additional NiII cations, and thus, the corresponding Hamiltonian to analyse the experimental data must be:
This expression is clearly overparameterised, and different solutions can be obtained assuming all constants or simplifying the Hamiltonian (in all cases R factor in the 10−5–10−6 range). This feature is a consequence of the topology of that complex: The butterfly subunit can give any local spin between S = 0 and S = 4 as function of the J 1 and J 2 values, and in addition, the two additional NiII cations form triangular subunits in which there are competitive interactions, and thus, only a qualitative analysis can be made.
It is well established that the FM/AF border for Ni–O–Ni bridges is placed around 96° for hydroxo bridges and values slightly larger for alkoxo or phenoxo ones. The most of the Ni–O–Ni bond angles in compound 2 lies around this border and thus, only weak ferro or anti-ferromagnetic interactions can be expected. In contrast, the very large Ni5-O22-Ni6 bond angle mediated by one of the O-donors of the carbonate ligand must give a clear AF interaction. In good agreement, all obtained fits gave a large dispersion of values for J 1–J 4, but in all cases, J 5 gave a value around −7 cm−1 (Scheme 4). In light of this data, we must assume that two S = 1 local spins are cancelled, whereas the remainder four spins are related by weak interactions.
The presence of competitive interactions is reinforced by magnetisation experiments. The unsaturated value under a field of 5 T is 6.25 Nμβ and can be reasonably fitted as an isolated S = 3 spin with D = 0.89 cm−1 and g = 2.14 (Fig. 3).
Conclusions
Our synthetic strategy to introduce NaI and carbonate, as co-ligand, along with H2L1 in Ni chemistry, resulted in two new Ni/Na compounds possessing a rare (for 1) and an unseen (for 2) topology. Magnetic studies reveal the presence of weak ferromagnetic interactions at low temperature for both compounds. Assumptions for the structural relationship of 1 and 2 can be attempted, but more synthetic studies are required to support it. Our future studies will be focused in two different directions: a) to extend the systematic synthetic study by varying metal ligand ratios and co-ligands aiming to achieve higher nuclearity CCs and b) to further develop our topological approach [101] by incorporating all centres as nodes.
References
Cronin L, Fielden J (2007) Coordination Clusters. In: Coord. Clust. Encycl. Supramol. Chem. Taylor Fr. London, 2007, Taylor & Francis, pp 1–10
Papatriantafyllopoulou C, Moushi EE, Christou G, Tasiopoulos AJ (2016) Filling the gap between the quantum and classical worlds of nanoscale magnetism: giant molecular aggregates based on paramagnetic 3d metal ions. Chem Soc Rev 45:1597–1628. doi:10.1039/c5cs00590f
Liu D-P, Lin X-P, Zhang H et al (2016) Magnetic properties of a single-molecule lanthanide-transition-metal compound containing 52 gadolinium and 56 nickel atoms. Angew Chem Int Ed Engl 55:4532–4536. doi:10.1002/anie.201601199
Peng J-B, Kong X-J, Zhang Q-C et al (2014) Beauty, symmetry, and magnetocaloric effect—four-shell keplerates with 104 lanthanide atoms. J Am Chem Soc 136:17938–17941. doi:10.1021/ja5107749
Schmidt S, Prodius D, Mereacre V et al (2013) Unprecedented chemical transformation: crystallographic evidence for 1,1,2,2-tetrahydroxyethane captured within an Fe(6)Dy(3) single molecule magnet. Chem Commun 49:1696–1698. doi:10.1039/c2cc38006d
Kitos AA, Efthymiou CG, Manos MJ et al (2016) Interesting copper(ii)-assisted transformations of 2-acetylpyridine and 2-benzoylpyridine. Dalton Trans 45:1063–1077. doi:10.1039/c5dt03912f
Perlepe PS, Cunha-Silva L, Gagnon KJ et al (2016) “Ligands-with-Benefits”: naphthalene-substituted schiff bases yielding new Ni(II) metal clusters with ferromagnetic and emissive properties and undergoing exciting transformations. Inorg Chem 55:1270–1277. doi:10.1021/acs.inorgchem.5b02492
Feltham HLC, Brooker S (2014) Review of purely 4f and mixed-metal nd-4f single-molecule magnets containing only one lanthanide ion. Coord Chem Rev 276:1–33. doi:10.1016/j.ccr.2014.05.011
Abtab SMT, Maity M, Bhattacharya K et al (2012) Syntheses, structures, and magnetic properties of a family of tetranuclear hydroxido-bridged Ni(II)2Ln(III)2 (Ln = La, Gd, Tb, and Dy) complexes: display of slow magnetic relaxation by the zinc(II)-dysprosium(III) analogue. Inorg Chem 51:10211–10221. doi:10.1021/ic301138r
Liu K, Shi W, Cheng P (2015) Toward heterometallic single-molecule magnets: synthetic strategy, structures and properties of 3d–4f discrete complexes. Coord Chem Rev 289–290:74–122. doi:10.1016/j.ccr.2014.10.004
Yang XP, Jones RA, Lynch V et al (2005) Synthesis and near infrared luminescence of a tetrametallic Zn2Yb2 architecture from a trinuclear Zn3L2 Schiff base complex. Dalton Trans. doi:10.1039/b416695g
Jankolovits J, Andolina CM, Kampf JW et al (2011) Assembly of near-infrared luminescent lanthanide host(host-guest) complexes with a metallacrown sandwich motif. Angew Chem Int Ed 50:9660–9664. doi:10.1002/anie.201103851
Samanta SK, Abtab SMT, Sardar PS et al (2014) Role of triplet states of two different ligands in the sensitized emission of LnIII (EuIII, TbIII) in d-f hybrid tetranuclear heterometal (ZnII2LnIII2, CdII2LnIII2) complexes. Eur J Inorg Chem. doi:10.1002/ejic.201402274
Jankolovits J, Kampf JW, Pecoraro VL (2014) Solvent dependent assembly of lanthanide metallacrowns using building blocks with incompatible symmetry preferences. Inorg Chem 53:7534–7546. doi:10.1021/ic500832u
Zhang L, Zhao L, Zhang P et al (2015) Nanoscale Ln(III)24Zn(II)6 triangular metalloring with magnetic refrigerant, slow magnetic relaxation, and fluorescent properties. Inorg Chem 54:11535–11541. doi:10.1021/acs.inorgchem.5b02215
Nesterov DS, Chygorin EN, Kokozay VN et al (2012) Heterometallic Co(III)4Fe(III)2 Schiff base complex: structure, electron paramagnetic resonance, and alkane oxidation catalytic activity. Inorg Chem 51:9110–9122. doi:10.1021/ic301460q
Okamura M, Kondo M, Kuga R et al (2016) A pentanuclear iron catalyst designed for water oxidation. Nature 530:465–468. doi:10.1038/nature16529
Powers TM, Betley TA (2013) Testing the polynuclear hypothesis: multielectron reduction of small molecules by triiron reaction sites. J Am Chem Soc 135:12289–12296. doi:10.1021/ja405057n
Manoli M, Alexandrou S, Pham L et al (2016) Magnetic “Molecular Oligomers” based on decametallic supertetrahedra: a giant Mn49 cuboctahedron and its Mn25 Na4 fragment. Angew Chem Int Ed 55:679–684. doi:10.1002/anie.201509461
Biswas R, Ida Y, Baker ML et al (2013) A new family of trinuclear nickel(II) complexes as single-molecule magnets. Chem Eur J 19:3943–3953. doi:10.1002/chem.201202795
Tong J, Demeshko S, John M et al (2016) Redox-induced single-molecule magnetism in mixed-valent [2 × 2] Co4 grid complexes. Inorg Chem 55:4362–4372. doi:10.1021/acs.inorgchem.6b00106
Cornia A, Gatteschi D, Sessoli R (2001) New experimental techniques for magnetic anisotropy in molecular materials. Coord Chem Rev 219:573–604
Wix P, Kostakis GE, Blatov VA et al (2013) A database of topological representations of polynuclear nickel compounds. Eur J Inorg Chem. doi:10.1002/ejic.201201348
Cadiou C, Murrie M, Paulsen C et al (2001) Studies of a nickel-based single molecule magnet: resonant quantum tunnelling in an S = 12 molecule. Chem Commun. doi:10.1039/b108894g
Bilyachenko AN, Yalymov AI, Korlyukov AA et al (2016) Unusual penta- and hexanuclear Ni(ii)-based silsesquioxane polynuclear complexes. Dalton Trans 45:7320–7327. doi:10.1039/c6dt00113k
Pait M, Bauzá A, Frontera A et al (2015) A new family of Ni4 and Ni6 aggregates from the self-assembly of [Ni2] building units: role of carboxylate and carbonate bridges. Inorg Chem 54:4709–4723. doi:10.1021/acs.inorgchem.5b00039
Keene TD, Hursthouse MB, Price DJ (2004) Ferromagnetic coupling in a heptanuclear nickel cluster with a vertex-shared dicubane structure. New J Chem 28:558–561. doi:10.1039/b315323a
Petit S, Neugebauer P, Pilet G et al (2012) Condensation of a nickel tetranuclear cubane into a heptanuclear single-molecule magnet. Inorg Chem 51:6645–6654. doi:10.1021/ic3001637
Bell A, Aromi G, Teat SJ et al (2005) Synthesis and characterisation of a Ni-8 single molecule magnet and another octanuclear nickel cage. Chem Commun. doi:10.1039/b500581g
Xiong K, Jiang F, Gai Y et al (2012) A series of octanuclear-nickel(II) complexes supported by thiacalix 4 arenes. Inorg Chem 51:3283–3288. doi:10.1021/ic202737h
Schmitz S, van Leusen J, Ellern A et al (2016) Thioether-terminated nickel(ii) coordination clusters with Ni 6 horseshoe- and Ni 8 rollercoaster-shaped cores. Inorg Chem Front 3:523–531. doi:10.1039/C5QI00278H
Scheurer A, Gieb K, Alam MS et al (2012) Synthesis, magnetic properties, and STM spectroscopy of an unprecedented octanuclear chloro-bridged nickel(II) double cubane. Dalton Trans 41:3553–3561. doi:10.1039/c2dt12007k
Hua S-A, Liu IP-C, Hasanov H et al (2010) Probing the electronic communication of linear heptanickel and nonanickel string complexes by utilizing two redox-active Ni-2(napy)(4) (3 +) moieties. Dalton Trans 39:3890–3896. doi:10.1039/b923125k
Xu J-Y, Song H-B, Xu G-F et al (2012) A new enneanuclear nickel(II) cluster with a rectangular face-centered trigonal prism structure and cluster glass behavior. Chem Commun 48:1015–1017. doi:10.1039/c1cc16243h
Ismayilov RH, Wang W-Z, Lee G-H et al (2011) Two linear undecanickel mixed-valence complexes: increasing the size and the scope of the electronic properties of nickel metal strings. Angew Chem Int Ed 50:2045–2048. doi:10.1002/anie.201006695
Brunet G, Habib F, Cook C et al (2012) A novel high-spin tridecanuclear Ni-II cluster with an azido-bridged core exhibiting disk-like topology. Chem Commun 48:1287–1289. doi:10.1039/c2cc16221k
Stamatatos TC, Escuer A, Abboud KA et al (2008) Unusual structural types in nickel cluster chemistry from the use of pyridyl oximes: Ni5, Ni12Na2, and Ni14 clusters. Inorg Chem 47:11825–11838. doi:10.1016/j.poly.2005.08.019
Gui L-C, Wang X-J, Ni Q-L et al (2012) Nanospheric M-20(OH)(12)(maleate)(12)(Me2NH)(12) (4 +) Clusters (M = Co, Ni) with O-h Symmetry. J Am Chem Soc 134:852–854. doi:10.1021/ja2100966
Fondo M, Ocampo N, Garca-deibe AM et al (2006) Self-assembly of a tetranuclear Ni cluster with an S = 4 ground state: the first 3d metal cluster bearing a μ4-η2:η2-O, O carbonate ligand. Inorg Chem 45(1):255–262. doi:10.1021/ic051194s
Dearden AL, Parsons S, Winpenny REP (2001) Synthesis, structure, and preliminary magnetic studies of a Ni-24 wheel. Angew Chem Int Ed 40:151–154. doi:10.1002/1521-3773(20010105)40:1<151:aid-anie151>3.3.co;2-j
Athanasopoulou AA, Pilkington M, Raptopoulou CP et al (2014) Structural aesthetics in molecular nanoscience: a unique Ni26 cluster with a “rabbit-face” topology and a discrete Ni18 “molecular chain”. Chem Commun 50:14942–14945. doi:10.1039/c4cc07192a
Nakajima T, Seto K, Horikawa F et al (2012) Wheel-shaped icosanuclear homo- and heterometallic complexes of Ni II, Co II, and Cu II ions supported by unsymmetrical aminoalcohol ligands. Inorg Chem 51:12503–12510. doi:10.1021/ic3019106
Andruh M (2015) The exceptionally rich coordination chemistry generated by Schiff-base ligands derived from o-vanillin. Dalton Trans 44:16633–16653. doi:10.1039/C5DT02661J
Bernardi A, Femoni C, Iapalucci MC et al (2008) Synthesis, molecular structure and properties of the [H6-nNi30C4(CO)34(CdCl)2]n- (n = 3–6) bimetallic carbide carbonyl cluster: a model for the growth of noncompact interstitial metal carbides. Chem Eur J 14:1924–1934. doi:10.1002/chem.200701519
Femoni C, Iapalucci MC, Longoni G, Svensson PH (2000) A high-nuclearity Ni–Sb carbonyl cluster displaying unprecedented metal stereochemistries: synthesis and X-ray structure of [NEt4]6[Ni31Sb4(CO)40]·2 Me2CO. Chem Commun. doi:10.1039/B000783H
Blake AJ, Grant CM, Parsons S et al (1994) The synthesis, structure and magnetic properties of a cyclic dodecanuclear nickel complex. Chem Commun. doi:10.1039/C39940002363
Chesman ASR, Turner DR, Moubaraki B et al (2009) Lanthaballs: chiral, structurally layered polycarbonate tridecanuclear lanthanoid clusters. Chem Eur J 15:5203–5207. doi:10.1002/chem.200900400
Chesman ASR, Turner DR, Moubaraki B et al (2012) Tetradecanuclear polycarbonatolanthanoid clusters: diverse coordination modes of carbonate providing access to novel core geometries. Dalton Trans 41:10903–10909. doi:10.1039/c2dt31101a
Escuer A, Vicente R, Kumar SB et al (1996) A novel pentadentate coordination mode for the carbonato bridge: synthesis, crystal structure, and magnetic behavior of (&mgr;(3)-CO(3))[Ni(3)(Medpt)(3)(NCS)(4)], a new trinuclear nickel(II) carbonato-bridged complex with strong antiferromagnetic coupling. Inorg Chem 35:3094–3098
Sakamoto S, Yamauchi S, Hagiwara H et al (2012) Carbonate-bridged tetranuclear NiII2GdIII2 complex generated by atmospheric CO2 fixation. Inorg Chem Commun 26:20–23. doi:10.1016/j.inoche.2012.08.022
Georgopoulou AN, Raptopoulou CP, Psycharis V et al (2009) Ferromagnetic Cu II4, Co II4, and Ni II6 Azido complexes derived from metal-assisted methanolysis of Di-2, 6- (2-pyridylcarbonyl) pyridine. Inorg Chem 48:3167–3176
Wikstrom JP, Filatov AS, Mikhalyova EA et al (2010) Carbonate formation within a nickel dimer: synthesis of a coordinatively unsaturated bis(μ-hydroxo) dinickel complex and its reactivity toward carbon dioxide. Dalton Trans 39:2504–2514. doi:10.1039/b916832j
Jonas K, Brauer DJ, Krüger C et al (1976) “Side-on” dinitrogen-transition metal complexes. J Am Chem Soc 98:74–81
Gole B, Mondal KC, Mukherjee PS (2014) Tuning nuclearity of clusters by positional change of functional group: synthesis of polynuclear clusters, crystal structures and magnetic properties. Inorg Chim Acta 415:151–164. doi:10.1016/j.ica.2014.02.017
Uehara K, Hikichi S, Inagaki A, Akita M (2005) Xenophilic complexes bearing a TpR ligand, [TpRM-M’Ln] [TpR = TpiPr2, Tp# (TpMe2,4-Br); M = Ni Co, Fe, Mn; M’Ln = Co(CO)4, Co(CO)3(PPh3), RuCp(CO)2]: the two metal centers are held together not by covalent interaction but by electrostatic attraction. Chem Eur J 11:2788–2809. doi:10.1002/chem.200401016
Monakhov KY, Lopez X, Speldrich M et al (2014) Magnetochemical complexity of hexa- and heptanuclear wheel complexes of late-3d ions supported by N, O-donor pyridyl-methanolate ligands. Chem Eur J 20:3769–3781. doi:10.1002/chem.201304177
Kermagoret A, Braunstein P (2008) Synthesis of nickel complexes with bidentate N, O-type ligands and application in the catalytic oligomerization of ethylene. Dalton Trans 32:1564–1573. doi:10.1039/b716111e
Botana L, Ruiz J, Mota AJ et al (2014) Anion controlled structural and magnetic diversity in unusual mixed-bridged polynuclear Ni(II) complexes with a versatile bis(2-methoxy phenol)diamine hexadentate ligand. An experimental and theoretical magneto-structural study. Dalton Trans 43:13509–13524. doi:10.1039/c4dt01253d
Brechin EK, Gilby LM, Gould RO et al (1998) Heterobimetallic nickel–sodium and cobalt–sodium complexes of pyridonate ligands. J Chem Soc Dalt Trans. doi:10.1039/a803442g
Cadiou C, Coxall RA, Graham A et al (2002) Octanuclear cobalt and nickel cages featuring formate ligands. Chem Commun. doi:10.1039/b202062a
Aromi G, Bell AR, Helliwell M et al (2003) A systematic exploration of nickel-pyrazolinato chemistry with alkali metals: new cages from serendipitous assembly. Chem Eur J 9:3024–3032. doi:10.1002/chem.200304939
Brechin EK, Graham A, Harris SG et al (1997) Overcrowding leads to prism reform: new polyhedra for polymetallic cages. J Chem Soc Dalton Trans. doi:10.1039/A704822J
Wang S, Bi Y, Liao W (2015) Constructing calixarene-supported high nuclearity Co27, Co28 and Ni18 Na6 clusters with triazoles as co-bridges. CrystEngComm 17:2896–2902. doi:10.1039/C5CE00314H
Su K, Jiang F, Qian J et al (2014) Generalized synthesis of calixarene-based high-nuclearity M4n nanocages (M = Ni or Co; n = 2–6). Cryst Growth Des 14:3116–3123
Stamatatos TC, Abboud KA, Perlepes SP, Christou G (2007) The highest nuclearity metal oxime clusters: Ni14 and Ni12Na2 complexes from the use of 2-pyridinealdoximate and azide ligands. Dalton Trans. doi:10.1039/b708189h
Chojnacki J, Oleksyn B, Zukowska E (1971) Prototype ligand with Uranium. Rocz Chem 45:487
Kannappan R, Tooke DM, Spek AL, Reedijk J (2006) Separation of actinides and lanthanides: synthesis and molecular structure of a new di-μ-phenoxo-bridged dinuclear bis(dioxouranium(VI)) complex. Inorg Chim Acta 359:334–338. doi:10.1016/j.ica.2005.08.020
Constable EC, Housecroft CE, Zampese JA, Zhang G (2012) Multinuclear zinc(II) complexes with {Zn6(μ-O)6(μ3-O)2}- and {Zn5(μ-O)3(μ3-O)3}-cluster cores. Polyhedron 44:150–155. doi:10.1016/j.poly.2012.06.033
Dey D, Kaur G, Ranjani A et al (2014) A trinuclear zinc-schiff base complex: biocatalytic activity and cytotoxicity. Eur J Inorg Chem. doi:10.1002/ejic.201402158
Hasanzadeh M, Salehi M, Kubicki M et al (2014) Synthesis, crystal structures, spectroscopic studies and antibacterial properties of a series of mononuclear cobalt(III) schiff base complexes. Trans Met Chem 39:623–632. doi:10.1007/s11243-014-9841-x
Saha S, Pal S, Gómez-García CJ et al (2014) A ferromagnetic tetranuclear nickel(II) Schiff-base complex with an asymmetric Ni4O4 cubane core. Polyhedron 74:1–5. doi:10.1016/j.poly.2014.02.036
Ke H, Zhu W, Zhang S et al (2015) A new family of heterometallic tetranuclear [MnIII2LnIII2] (Ln = Eu, Gd, Tb, Dy) isostructural clusters: syntheses, crystal structures and magnetic properties. Polyhedron 87:109–116. doi:10.1016/j.poly.2014.10.030
Ke H, Zhang S, Zhu W et al (2015) Synthesis, structures and magnetic properties of four dodecanuclear Ni8RE4 (RE = Gd, Dy, Y) clusters trapping four μ 5-bridged carbonate anions. J Coord Chem 68:808–822. doi:10.1080/00958972.2015.1004326
Kuang W-W, Shao C-Y, Yang P-P (2015) Syntheses, crystal structures, and magnetic properties of a series of Fe2Ln complexes. J Coord Chem 68:1412–1422. doi:10.1080/00958972.2015.1013947
Griffiths K, Novitchi G, Kostakis GE (2016) Synthesis, characterization, magnetic properties, and topological aspects of isoskeletal heterometallic hexanuclear Co II 4 Ln III 2 coordination clusters possessing 2,3,4M6-1 topology. Eur J Inorg Chem. doi:10.1002/ejic.201600078.10.1002/ejic.201600078
Griffiths K, Gallop CWD, Abdul-Sada A et al (2015) Heteronuclear 3 d/Dy(III) coordination clusters as catalysts in a domino reaction. Chem Eur J 21:6358–6361. doi:10.1002/chem.201500505
Ruiz J, Lorusso G, Evangelisti M et al (2014) Closely-related Zn(II)2Ln(III)2 complexes (Ln(III) = Gd, Yb) with either magnetic refrigerant or luminescent single-molecule magnet properties. Inorg Chem 53:3586–3594. doi:10.1021/ic403097s
Keypour H, Rezaeivala M, Ramezani-Aktij A et al (2016) New macrocyclic schiff base complexes incorporating a homopiperazine unit: synthesis of some Co(II), Ni(II), Cu(II) and Zn(II) complexes and crystal structure and theoretical studies. J Mol Struct 1115:180–186. doi:10.1016/j.molstruc.2016.02.071
Coles SJ, Gale PA (2012) Changing and challenging times for service crystallography. Chem Sci 3:683–689. doi:10.1039/c2sc00955b
Dolomanov OV, Blake AJ, Champness NR, Schröder M (2003) OLEX: new software for visualization and analysis of extended crystal structures. J Appl Crystallogr 36:1283–1284
Palatinus L, Chapuis G (2007) SUPERFLIP—a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J Appl Crystallogr 40:786–790. doi:10.1107/S0021889807029238
Farrugia LJ (1999) Suite for small-molecule single-crystal crystallography. J Appl Crystallogr 32:837–838. doi:10.1107/S0021889899006020
Sheldrick GM (2015) SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr Sect A Found Adv 71:3–8. doi:10.1107/S2053273314026370
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr Sect A 64:112–122. doi:10.1107/s0108767307043930
Spek AL (2003) Single-crystal structure validation with the program PLATON. J Appl Crystallogr 36:7–13. doi:10.1107/S0021889802022112
Macrae CF, Edgington PR, McCabe P et al (2006) Mercury: visualization and analysis of crystal structures. J Appl Crystallogr 39:453–457. doi:10.1107/S002188980600731X
Nemec I, Machata M, Herchel R et al (2012) A new family of Fe2Ln complexes built from mononuclear anionic schiff base subunits. Dalton Trans 41:14603–14610. doi:10.1039/c2dt31809a
Mondal KC, Kostakis GE, Lan Y et al (2011) Defect-dicubane Ni2Ln2 (Ln = Dy, Tb) single molecule magnets. Inorg Chem 50:11604–11611. doi:10.1021/ic2015397
Mondal KC, Sundt A, Lan Y et al (2012) Coexistence of distinct single-ion and exchange-based mechanisms for blocking of magnetization in a Co(II)2Dy(III)2 single-molecule magnet. Angew Chem Int Ed 51:7550–7554. doi:10.1002/anie.201201478
Mondal KC, Kostakis GE, Lan Y, Powell AK (2013) Magnetic properties of five planar defect dicubanes of [LnIII4(μ3-OH)2(L)4(HL)2]·2THF (Ln = Gd, Tb, Dy, Ho and Er). Polyhedron 66:268–273. doi:10.1016/j.poly.2013.05.016
Ke H, Zhao L, Guo Y, Tang J (2012) Syntheses, structures, and magnetic analyses of a family of heterometallic hexanuclear [Ni4M2] (M = Gd, Dy, Y) compounds: observation of slow magnetic relaxation in the Dy(III) derivative. Inorg Chem 51:2699–2705. doi:10.1021/ic202699k
Kostakis GE, Blatov VA, Proserpio DM (2012) A method for topological analysis of high nuclearity coordination clusters and its application to Mn coordination compounds. Dalton Trans 41:4634–4640. doi:10.1039/c2dt12263d
Zhang K, Kurmoo M, Wei LQ, Zeng MH (2013) iterative mass spectrometry and X-ray crystallography to study ion-trapping and rearrangements by a flexible cluster. Sci Rep 3: Article number: 3516. doi: Artn 3516\rDoi 10.1038/Srep03516
Dubey M, Koner RR, Ray M (2009) Sodium and potassium ion directed self-assembled multinuclear assembly of divalent nickel or copper and l-leucine derived ligand. Inorg Chem 48:9294–9302. doi:10.1021/ic9011444
Bhula R, Weatherburn DC (1991) Oxidative cleavage of triethylenetetramine (trien) to yield dietheylenetriamine (dien): structure of the MnII/MnIII heptanuclear complex [Mn7(trien)2(dien)2O4(OAc)8](PF6)4 2H2O. Angew Chem Int Ed 30:688–689
Wang S, Tsai H-L, Streib WE et al (1992) High nuclearity molecular species exhibiting spin frustration: fusion of two MnIII 4O2 butterfly complexes to yield an intermediate spin ground state MnIII 7O4 complex. J Chem Soc Chem Commun. doi:10.1039/C39920000677
Krautscheid H, Vielsack F (1999) Discrete and polymeric iodoplumbates with Pb3I10 building blocks: [Pb3I10] 4_, [Pb7I22] 8_, [Pb10I28] 8_, 1 ∞[Pb3I10] 4_ and 2 ∞[Pb7I18] 4_. Dalton Trans. doi:10.1007/3-540-44628-1_13
Ding C, Zeng F, Ni J et al (2012) Polynuclear complexes of ligands containing in situ formed oxazinane and oxazolidine rings with appended alkoxyl and phenol groups. Cryst Growth Des 12:2089–2096. doi:10.1021/cg300096n
Tarushi A, Kastanias F, Psycharis V et al (2012) A [24-MC-6] zinc metallacoronate with a nonsteroidal antiinflammatory drug as the constructing ligand. Inorg Chem 51:7460–7462. doi:10.1021/ic3010757
Chilton NF, Anderson RP, Turner LD et al (2013) PHI: a powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J Comput Chem 34:1164–1175. doi:10.1002/jcc.23234
Blatov VA, Shevchenko AP, Proserpio DM (2014) Applied topological analysis of crystal structures with the program package ToposPro. Cryst Growth Des 14:3576–3586. doi:10.1021/cg500498k
Acknowledgments
We thank the EPSRC UK National Crystallography Service at the University of Southampton for the collection of the crystallographic data for compounds 1 and 2. G. E. K. acknowledges the University of Sussex for offering a Ph.D. position to K. G. A. E. thanks for the support from the Comisión Interministerial de Ciencia y Tecnología (CICYT) under project CTQ2015-63614-P.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is dedicated to Prof Vladimir Ya. Shevcehnko on the occasion of his 75th birth day.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Griffiths, K., Escuer, A. & Kostakis, G.E. Topological insights in polynuclear Ni/Na coordination clusters derived from a schiff base ligand. Struct Chem 27, 1703–1714 (2016). https://doi.org/10.1007/s11224-016-0797-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0797-7