Abstract
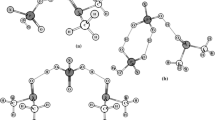
Proton transfer processes in various H-bonded complexes of phosphoric (H3PO4), phosphorous (H3PO3) and methylphosphonic (H2MePO3) acids with dimethyl sulfoxide (DMSO), i.e., (acid)2–DMSO, acid–(DMSO) n for n = 1, 2 and H3PO4–(DMSO)3 have been studied. The potential energy surface (PES) for proton transfer was investigated using the B3LYP level of theory, and the solvent effect on the PES was included using the conductor polarized continuum model. The energy curves for proton transfer from acid to DMSO oxygen atom in all investigated complexes represent single-well potentials, if no constraints are imposed on the system. The PES obtained by optimizing the geometry of each complex at fixed O···O distances has two nonsymmetric minima with respect to the energy barrier. In these cases, the distance at which a barrier starts to rise is slightly different. The energy barrier for proton transfer in all considered complexes increases in the series of acids: H3PO4 < H3PO3 < H2MePO3. The energies associated with proton transfer in the complexes of all investigated acid with DMSO become higher with increasing number of DMSO molecules. For all complexes, the effect of DMSO environment favors a proton transfer.




Similar content being viewed by others
References
Jeffrey GA, Saenger W (1991) Hydrogen bonding in biological structures. Springer, Berlin
Bell RP (1980) The tunnel effect in chemistry. Chapman and Hall, New York
Melander L, Saunders WH (1980) Reaction rates of isotopic molecules. Wiley, New York
Bender ML (1971) Mechanisms of homogeneous catalysis from protons to proteins. Wiley, New York
Basilevsky MV, Vener MV (2003) Theoretical investigations of proton and hydrogen atom transfer in the condensed phase. Russ Chem Rev 72:1–33
Sobczyk L, Czarnik-Matusewicz B, Rospenk M, Obrzud M (2012) Proton transfer equilibria and critical behavior of H-bonding. J Atom Mol Opt Phys 217932:1–10
Szafran M (1996) Recent aspects of the proton transfer reaction in H-bonded complexes. J Mol Struct 381:39–64
Vilčiauskas L, Tuckerman ME, Melchior JP, Bester G, Kreuer K-D (2013) First principles molecular dynamics study of proton dynamics and transport in phosphoric acid/imidazole (2:1) system. Solid State Ion 252:34–39
Fadeeva JA, Safonova LP, Persson I (2010) Physico-chemical and structural characterization of the binary system phosphoric acid–N,N-dimethylformamide. Phys Chem Chem Phys 12:8977–8984
Li S, Fried JR (2013) Ab initio study of proton transfer and interfacial properties in phosphoric acid-doped polybenzimidazole. Macromol Theory Simul 22:410–425
Shirata K, Kawauchi S (2015) Effect of benzimidazole configuration in polybenzimidazole chain on interaction with phosphoric acid: a DFT study. J Phys Chem B 119:592–603
Kreuer K-D (1996) Proton conductivity: materials and applications. Chem Mater 8:610–641
Sata T, Yoshida T, Matsusaki K (1996) Transport studies of phosphonic acid and sulfonic acid cation exchange membranes. J Membr Sci 120:101–110
Schuster M, Rager T, Noda A, Kreuer KD, Maier J (2005) About the choice of the protogenic group in PEM separator materials for intermediate temperature, low humidity operation: a critical comparison of sulfonic acid, phosphonic acid and imidazole functionalized model compounds. Fuel Cells 5:355–365
Munson RA (1964) Self-dissociative equilibria in molten phosphoric acid. J Phys Chem 68:3374–3377
Dippel T, Kreuer KD, Lassègues JC, Rodriguez D (1993) Proton conductivity in fused phosphoric acid: a 1H/31P PFG-NMR and QNS study. Solid State Ion 61:41–46
Vilčiauskas L, Tuckerman ME, Bester G, Paddison SJ, Kreuer K-D (2012) The mechanism of proton conduction in phosphoric acid. Nat Chem 4:461–466
Aihara Y, Sonai A, Hattori M, Hayamizu K (2006) Ion conduction mechanisms and thermal properties of hydrated and anhydrous phosphoric acids studied with 1H, 2H, and 31P NMR. J Phys Chem B 110:24999–25006
Schuster M, Kreuer K-D, Steininger H, Maier J (2008) Proton conductivity and diffusion study of molten phosphonic acid H3PO3. Solid State Ion 179:523–528
Vilciauskas L, Paddison SJ, Kreuer K-D (2009) Ab initio modeling of proton transfer in phosphoric acid clusters. J Phys Chem A 113:9193–9201
Krest’yaninov MA, Kiselev MG, Safonova LP (2015) Ab initio calculations of proton transfer in dimethylformamide-phosphoric acid complexes of 1:1 composition. Russ J Phys Chem A 89:608–615
Joswig J-O, Hazebroucq S, Seifert G (2007) Properties of the phosphonic-acid molecule and the proton transfer in the phosphonic-acid dimer. J Mol Struct Theochem 816:119–123
Li T, Wlaschin A, Balbuena PB (2001) Theoretical studies of proton transfer in water and model polymer electrolyte systems. Ind Eng Chem Res 40:4789–4800
Fedorova IV, Safonova LP (2016) Influence of solvent environment using the CPCM model on the H-bond geometry in the complexes of phosphorus acids with DMSO. Struct Chem. doi:10.1007/s11224-016-0744-7
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24:669–681
Khatuntseva EA, Krest’yaninov MA, Fedorova IV, Safonova LP (2015) Hydrogen bonds in complexes of phosphonic and metylphosphonic acids with dimethylformamide. Russ J Phys Chem A 89:2248–2253
Fedorova IV, Khatuntseva EA, Krest’yaninov MA, Safonova LP (2016) C-PCM based calculation of energy profiles for proton transfer in phosphorus-containing acid–N,N-dimethylformamide complexes. Russ J Phys Chem A 90:293–299
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 revision A.01. Gaussian, Inc., Wallingford
Riddick JA, Bunger WB (1970) Organic solvents, vol. II of techniques of organic chemistry, 3rd edn. Wiley-Interscience, New York
Kreuer KD (2000) On the complexity of proton conduction phenomena. Solid State Ion 136–137:149–160
Morrone JA, Haslinger KE, Tuckerman ME (2006) Ab initio molecular dynamics simulation of the structure and proton transport dynamics of methanol-water solutions. J Phys Chem B 110:3712–3720
Shun-Li O, Nan-Nan W, Jing-Yao L, Cheng-Lin S, Zuo-Wei L, Shu-Qin G (2010) Investigation of hydrogen bonding in neat dimethyl sulfoxide and binary mixture (dimethyl sulfoxide + water) by concentration-dependent Raman study and ab initio calculation. Chin Phys B 19:123101–123107
Acknowledgments
The authors acknowledge the support from the Russian Foundation for Basic Research (Nos. 14-03-00481, 15-43-03088).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fedorova, I.V., Safonova, L.P. Proton transfer in the molecular complexes of phosphorus acids with DMSO. Struct Chem 27, 1561–1567 (2016). https://doi.org/10.1007/s11224-016-0786-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0786-x