Abstract
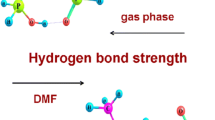
Quantum chemical calculations of structure and energies of various H-bonded complexes of phosphoric, phosphorous and methylphosphonic acids and their dimers with dimethylsulfoxide (DMSO), i.e., (acid) n –DMSO and acid–(DMSO) m for n = 1, 2 and m = 2, 3 have been carried out. The polar solvent effect is taken into account by using the CPCM model. It has been found that in DMSO environment the H-bonds in all complexes of investigated acid with DMSO are sizably stronger than the ones in the gas phase. At B3LYP-CPCM computation, the H-bonds between all investigated acid dimers and DMSO are significantly shorter than those found for complexes of corresponding acids with other compositions. The H-bonding interaction in acid–(DMSO) m for m = 1–3 becomes slightly weaker with increasing number DMSO molecules. The strength of the H-bond in all investigated complexes increases in the series of acids: (HO)2MePO < (HO)2P(O)H < H3PO4. Additionally, quantum theory of ‘atoms in molecules’ and natural bond orbitals method have been applied to analyze H-bond interactions.


Similar content being viewed by others
References
Korbridge DEC (1985) Phosphorus—an outline of its chemistry, biochemistry and technology, 3rd edn. Elsevier, Amsterdam
Kreuer KD (1996) Chem Mater 8:610–641
Munson RA (1964) J Phys Chem 68:3374–3377
Lagier CM, Zuriaga M, Monti G et al (1996) J Phys Chem Solids 57:1183–1190
Ewig CS, Van Wazer JR (1985) J Am Chem Soc 107:1965–1971
Yekutiel M, Lane JR, Gupta P et al (2010) J Phys Chem A 114:7544–7552
Range K, McGrath MJ, Lopez X et al (2004) J Am Chem Soc 126:1654–1665
Fedorova IV, Krishtal SP, Kiselev MG, Safonova LP (2006) Russ J Phys Chem 80:S7–S13
Heggen B, Roy S, Müller-Plathe F (2008) J Phys Chem 112:14209–14215
Joswig J-O, Hazebroucq S, Seifert G (2007) J Mol Struct Theochem 816:119–123
Khatuntseva EA, Krest’yaninov MA, Fedorova IV et al (2015) Russ J Phys Chem A 89:2264–2269
Sullivan PA, Sumathi R, Green WH, Tester JW (2004) Phys Chem Chem Phys 6:4296–4309
Vilciauskas L, Paddison SJ, Kreuer K-D (2009) J Phys Chem A 113:9193–9201
Paddison SJ, Kreuer K-D, Maier J (2006) Phys Chem Chem Phys 8:4530–4542
Kraikin LS, Grikina OE, Vilkov LV et al (2003) J Mol Struct 658:153–170
Yue B, Yan L, Han S, Xie L (2013) J Phys Chem B 117:7941–7949
Gonzalez L, Mo O, Yanez M, Elguero J (1998) J Chem Phys 109:2685–2693
Pereira RP, Felisberti MI, Rocco AM (2006) Polymer 47:1414–1422
Hossain MA, Isiklan M, Pramanik A et al (2012) Cryst Growth Des 12:567–571
Kołaski M, Cho SJ (2012) Bull Korean Chem Soc 33:1998–2004
Park SW, Kim CW, Lee JH et al (2011) J Phys Chem A 115:11355–11361
Wilson CC, Morrison CA (2002) Chem Phys Lett 362:85–89
Krawietz TR, Lin P, Lotterhos KE et al (1998) J Am Chem Soc 120:8502–8511
Zhang D, Yan L (2010) J Phys Chem B 114:12234–12241
Ilczyszyn MM (2002) J Mol Struct 611:119–129
Ilczyszyn MM, Ratajczak H (1996) J Mol Struct 375:213–222
Krest’yaninov MA, Kiselev MG, Safonova LP (2012) Russ J Phys Chem A 86:1847–1854
Fedorova IV, Krestyaninov MA, Kiselev MG, Safonova LP (2016) J Mol Struct 1106:424–429
Cossi M, Rega N, Scalmani G, Barone V (2003) J Comp Chem 24:669–681
Riddick JA, Bunger WB (1970) Organic solvents, vol. II of techniques of organic chemistry, 3rd edn. Wiley-Interscience, New York
Shun-Li O, Nan-Nan W, Jing-Yao L et al (2010) Chin Phys B 19:123101–123107
Steiner T (2002) Angew Chem Int Ed 41:48–76
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Weinhold F, Landis CR (2005) Valency and bonding. A natural bond orbital donor—acceptor perspective. Cambridge University Press, Cambridge
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09, Revision A.01. Gaussian Inc., Wallingford
Koch W, Holthausen MC (2001) A Chemist’s guide to density functional theory, 2nd edn. Wiley-VCH, Weinheim
Scheiner AC, Baker J, Andzelm JW (1997) J Comput Chem 18:775–795
Sharma A, Ohanessian G, Clavaguéra C (2014) J Mol Model 20:2426–2434
Blessing RH (1988) Acta Cryst B44:334–340
Souhassou M, Espinosa E, Lecompte C, Blessing RH (1995) J Acta Cryst B 51:661–668
Furberg S (1955) Acta Chem Scand 9:1557–1566
Tromp RH, Spieser SH, Neilson GW (1999) J Chem Phys 110:2145–2150
Furberg S, Landmark P (1957) Acta Chem Scand 11:1505–1511
Becker G, Hausen H-D, Mundt O et al (1990) Z Anorg Allg Chem 591:17–31
Reuter H, Reichelt M (2014) Acta Cryst E 70:o353
Thomas R, Shoemaker CB, Eriks K (1966) Acta Cryst 21:12–20
Frolov YL, Guchik IV, Shagun VA et al (2003) J Struct Chem 44:927–931
Shun-Li O, Nan-Nan W, Jing-Yao L et al (2010) Chin Phys B 19:123101(7)
Van Duijneveldt FB, Van Duijneveldt-van JGCM, Van Lenthe JH (1994) Chem Rev 94:1873–1885
Keith TA (2010) AIMAll (Version 10.05.04) (aim.tkgristmill.com)
Weinhold F (1997) J Mol Struct Theochem 398–399:181–197
Koch U, Popelier PLA (1995) J Chem Phys 99:9747–9754
Popelier PLA (1998) J Phys Chem A 102:1873–1878
Lyssenko KA, Antipin MYu (2006) Russ Chem Bull Int Ed 55:1–15
Espinosa E, Molins E, Lecomte C (1998) J Chem Phys Lett 285:170–173
Safonova LP, Fadeeva YA, Pryakhin AA (2009) Russ J Phys Chem 83:1747–1750
Sprik M, Hutter J, Parrinello M (1996) J Chem Phys 105:1142–1152
Acknowledgments
This work was financially supported by the Russian Foundation for Basic Research (Project No. 15-43-03088).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fedorova, I.V., Safonova, L.P. Influence of solvent environment using the CPCM model on the H-bond geometry in the complexes of phosphorus acids with DMSO. Struct Chem 27, 1189–1198 (2016). https://doi.org/10.1007/s11224-016-0744-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0744-7