Abstract
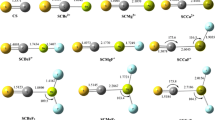
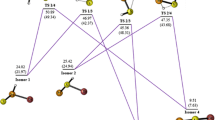
Ab initio and density functional theory (DFT) calculations are employed to investigate the formation and isomerization pathways of methyl thionitrate, CH3SNO2, and the S–N bond dissociation energies (BDE) of the sulfonated methyl thionitrates of the type CH3S(O) n NO2 (n = 0, 1, 2). The calculations indicate that CH3SNO2 may be formed as a stabilized by-product in the reaction CH3S + NO2 which mainly leads to CH3SO + NO. In general, the compounds of the type CH3S(O) n NO2 (n = 0, 1, 2) may be formed as bound intermediates in the homologous reactions CH3S(O) n + NO2. The calculated S–N BDE exhibit an interesting dependence on the number of the sulfonic oxygen atoms present in each species.





Similar content being viewed by others
References
Arsene C, Barnes I, Becker KH (1999) Phys Chem Chem Phys 1:5463
Finlayson-Pitts BJ, Pitts JN Jr (2000) Chemistry of the upper and lower atmosphere. Academic, New York
Arsene C, Barnes I, Becker KH, Mocanu R (2001) Atmos Environ 35:3769
Atkinson R, Baulch DL, Cox RA, Crowley JN, Hampson RF, Hynes RG, Jenkin ME, Rossi MJ, Troe J (2004) Atmos Chem Phys 4:1461
Barnes I, Hjorth J, Mihalopoulos N (2006) Chem Rev 106:940
Butkovskaya NI, LeBras G (1994) J Phys Chem 98:2582
Turnipseed AA, Barone SB, Ravishankara AR (1996) J Phys Chem 100:14703
Urbanski SP, Stickel RE, Zhao Z, Wine PH (1997) J Chem Soc Faraday Trans 93:2813
Barnes I, Bastian V, Becker KH, Niki H (1987) Chem Phys Lett 140:451
Jensen NR, Hjorth J, Lohse C, Scov H, Restelli G (1992) J Atmos Chem 14:95
Turnipseed AA, Barone SB, Ravishankara AR (1993) J Phys Chem 97:5926
Martinez E, Albaladejo J, Jimenez E, Notario A, Aranda A (1999) Chem Phys Lett 308:37
Chang P-F, Wang TT, Wang NS, Hwang Y-L, Lee Y-P (2000) J Phys Chem A 104:5525 (references therein)
Stamler JS, Singel DJ, Loscalzo J (1992) Science 258:1898
Lipton SA, Choi YB, Pan ZH, Lei SZZ, Chen HSV, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS (1993) Nature 364:895
Cameron DR, Borrajo AMP, Bennett BM, Thatcher GRJ (1995) Can J Chem 73:1627
Parker JD, Gori T (2001) Circulation 104:2263
Thacher GRJ, Nicolescu AC, Bennet BM, Toader V (2004) Free Radic Biol Med 37:1122
Clarke JL, Kastrati I, Johnston LJ, Thatcher GRJ (2006) Can J Chem 84:709
Tang YZ, Sun H, Pan YR, Pan XM, Wang RS (2006) Int J Quan Chem 107:1495
Gonzales C, Schlegel HB (1989) J Chem Phys 90:2154
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda KR, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Straroverov VN, Kobayashi R, Normand J, Raghavashari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo VC, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski J, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 Inc. Gaussian, Wallingford
McKee ML (1994) Chem Phys Lett 231:257
Lacombe S, Loudet M, Cardy H, Dargelos A (1999) Chem Phys 244:175
Resende SM, De Almeida WB (1999) Phys Chem Chem Phys 1:2953
Zhu L, Bozzelli JS (2006) J Phys Chem A 110:6923
Kukui A, Bossoutrot V, Laverdet G, Le Bras G (2000) J Phys Chem A 104:935
Lesar A (2012) Int J Quant Chem 112:1904
Borissenko D, Kukui A, Laverdet G, Le Bras G (2003) J Phys Chem A 107:1155
Ray A, Vassalli I, Laverdet G, Le Bras G (1996) J Phys Chem 100:8895
Patroescu IV, Barnes I, Becker KH, Mihalopoulos N (1999) Atmos Environ 33:25
Salta Z, Kosmas AM (2014) Int J Quant Chem 114:1430
Atak K, Engel N, Lange KM, Golnak R, Gotz M, Soldatov M, Rubersson JE, Kosug N, Aziz EF (2012) Chem Phys Chem 13:3106
Lindquist BA, Takeshita TY, Woon DE, Dunning TH Jr (2013) J Chem Theory Comput 9:4444
Acknowledgments
This research has been co-financed by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund. Computer services provided by the Center of Molecular Simulations of the University of Ioannina are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salta, Z., Kosmas, A.M. Computational investigation of the formation and isomerization pathways of CH3SNO2 and the S–N bond dissociation energies of CH3S(O) n NO2 (n = 0, 1, 2) species. Struct Chem 27, 1149–1156 (2016). https://doi.org/10.1007/s11224-015-0737-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0737-y