Abstract
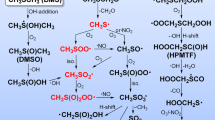
Products and mechanisms of the reaction between the nitrate radical (NO3) and three of the most abundant reduced organic sulphur compounds in the atmosphere (CH3SCH3, CH3SH and CH3SSCH3), have been studied in a 480 L reaction chamber using in situ FT-IR and ion chromatography as analytical techniques. In the three reactions, methanesulphonic acid was found to be the most abundant sulphur containing product. In addition the stable products SO2, H2SO4, CH2O, and CH3ONO2 were identified and quantified and thionitric acid-S-methyl ester (CH3SNO2) was observed in the i.r. spectrum from all of the three reactions. Deuterated dimethylsulphide (CD3SCD3) showed an isotope effect on the reaction Deuterated dimethylsulphide (CD3SCD3) showed an isotope effect on the reaction rate constant (kH/kD) of 3.8±0.6, indicating that hydrogen abstraction is the first step in the NO3+CH3SCH3 reaction, probably after the formation of an inital adduct.
Based on the products and intermediates identified, reaction mechanisms are proposed for the three reactions.
Similar content being viewed by others
References
Andreae M.O. and Raemdonck H., 1983, Dimethylsulfide in the surface ocean and the marine atmosphere: A global view,Science,221, 744–747.
Atkinson R., Pitts J. N.Jr. and Aschmann S. M., 1984, Tropospheric reactions of dimethyl sulphide with NO3 and OH radicals.,J. Phys. Chem.,88, 1584–1587.
Atkinson R., Baulch D. L., Cox R. A., Hampson R. F.Jr., Kerr J. A. and Troe J., 1989, Evaluated kinetic and photochemical data for atmospheric chemistry: Supplement III,J. Phys Chem. Ref. Data.,18, 881–1096.
Balla R. J., Nelson H. H. and McDonald J. R., 1986, Kinetics of the reaction of CH3S with NO, NO2 and O2.,Chem. Phys.,109, 101–107.
Barnes I., Bastian V., Becker K. H. and Wirtz K., 1987a, Atmospheric sulfur compounds: Source and tropospheric oxidation processes.,Dechema-Monographs,104, 59–77.
Barnes I., Bastian V., Becker K. H. and Niki H., 1987b, FTIR spectroscopic studies of the CH3S+NO2 reaction under atmospheric conditions.,Chem. Phys. Lett.,140, 451–457.
Barnes I., Bastian V. and Becker K. H., 1988, Kinetic and mechanisms of the reaction of OH radicals with dimethyl sulfide.,Int. J. Chem. Kinet.,20, 415–431.
Charlson R. J., Lovelock J. E., Andreae M. O. and Warren S. G. 1987, Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate.,Nature,326, 655–661.
Daykin E. P. and Wine P. H., 1990, A study of the reactions of NO3 radicals with organic sulfides: Reactivity trends at 298 K,Int. J. Chem. Kinet.,22, 1083–1094.
Dlugokencky E. J. and Howard C. J., 1988, Laboratory studies of the NO3 radical reactions with some atmospheric sulphur compounds.,J. Phys. Chem.,92, 1188–1193.
Domine' F., Murrells T. P. and Howard C. J., 1990, Kinetic of the reactions of NO2 with CH3S, CH3SO, CH3SS and CH3SSO at 297 K and 1 Torr.,J. Phys Chem.,94, 5839–5847.
Finlayson-Pitts B. J. and Pitts J. N.Jr., 1986,Atmospheric Chemistry, John Wiley, New York.
Hatakeyama S. and Akimoto H., 1983, Reaction of OH radical with methanethiol, dimethyl sulphide, and dimethyl disulphide in air.,J. Phys Chem.,87, 2387–2395.
Hatakeyama S., Izumi K. and Akimoto H., 1985, Yield of SO2 and formation of aerosol in the photo-oxidation of DMS under atmospheric conditions.,Atmospheric Environment,19, 135–141.
Jensen, N. R., Hjorth, J., Lohse, C., Skov, H. and Restelli, G., 1991, Products and mechanism of the reaction between NO3 and dimethylsulphide in air.,Atmospheric Environment, In press.
Mac Leod H., Aschmann S. M., Atkinson R., Tuazon E. C., Sweetman J. A., Winer A. M. and Pitts J. N.Jr., 1986, Kinetic and mechanism of the gas phase reaction of the NO3 radical with a series of reduced sulfur compounds.,J. Geophys. Res.,91, 5338–5346.
Mellouki A., Jourdain J. L. and Le Bras G., 1988, Discharge flow study of the CH3S+NO2 reaction mechanism using CL + CH3SH as the source.,Chem. Phys. Lett.,148, 231–236.
Niki H., Marker P. D., Savage C. M. and Breitenbach L. P., 1983a, An FTIR study of the mechanism for the reaction OH+CH3SCH3.,Int. J. Chem. Kinet.,15, 647–654.
Niki H., Marker P. D., Savage C. M. and Breitenbach L. P., 1983b, Spectroscopic and photochemical properties of CH3SNO.,J. Phys. Chem.,87, 7–9.
Notholt, H., Unpublished result.
Platt U., Le Bras G., Poulet G., Burrows J. P. and Moortgat G., 1990, Peroxy radicals from the night-time reaction of NO3 with organic compounds.,Nature,348, 147–149.
Saltzman, E. S. and Cooper, W. J., 1989,Biogenic Sulfur in the Environment, ACS Symposium series 393.
Scott G. and Davidson N., 1958, Shock waves in chemical kinetics: The decomposition of N2O5 at high temperature.,J. Amer. Chem. Soc.,80, 1841–1853.
Tyndall, G. S., Burrows, J. P., Schneider, W., Bingemer, H. and Moortgat., 1985, Paper presented at Workshop on “Chemistry related to tropospheric ozone”, Cost 611 (working party 2), Cologne, 12–13 November 1985.
Tyndall G. S. and Ravishankara A. R., 1989, Kinetic and mechanism of the reactions of CH3S with O2 and NO2 at 298 K.,J. Phys. Chem.,93, 2426–2435.
Wallington T. J., Atkinson R., Winer A. M. and Pitts J. N.Jr., 1986, Absolute rate constants for the gas-phase reactions of the NO3 radical with CH3SH, CH3SCH3, CH3SSCH3, H2S, SO2 and CH3OCH3 over the temperature range 280–350 K.,J. Phys. Chem.,90, 5393–5396.
Wayne R. P., Barnes I., Biggs P., Burrows J. P., Canosa-Mas C. E., Hjorth J., Le Bras G., Moortgat G., Perner D., Poulet G., Restelli G. and Sidebottom H., 1991 The nitrate radical: Physics, chemistry and atmosphere.,Atmospheric Environment,25, 1–250.
Yin F., Grosjean D. and Seinfeld J. H., 1986, Analysis of atmospheric photooxidation mechanisms for organosulfur compounds.,J. Geophys. Res.,91, 14417–14438.
Zabel F., Reimer A., Becker K. H. and Fink E. H., 1989, Thermal decomposition of alkyl peroxynitrates.,J. Phys. Chem.,93, 5500–5507.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jensen, N.R., Hjorth, J., Lohse, C. et al. Products and mechanisms of the gas phase reactions of NO3 with CH3SCH3, CD3SCD3, CH3SH and CH3SSCH3 . J Atmos Chem 14, 95–108 (1992). https://doi.org/10.1007/BF00115226
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00115226