Abstract
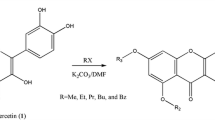
Synthesis of glycidyl ethers of quercetin and studies of their structure and spectral properties have been carried out. Using FTIR spectroscopy, 1H and HSQC NMR spectroscopy, mass spectrometry and quantum chemical simulations, it was shown that glycidation of 7-hydroxy and 4′-hydroxy groups primarily takes place, and then, the sequential glycidation of 3′-hydroxy and, further, 3-hydroxy groups occurs. Solvatochromic effects and quadratic polarizability of the obtained ethers—7,4′-diglycidyloxy-3,5,3′-trihydroxyflavone, 7,3′,4′-triglycidyloxy-5,3′-dihydroxyflavone and 3,7,3′,4′-tetraglycidyloxy-5-hydroxyflavone—were studied. It was shown that the diglycidyl and triglycidyl ethers can be used for creation of new polymeric NLO materials.





Similar content being viewed by others
References
Wu J, Xiao H, Qiu L, Zhen Zh, Liub X, Bo Sh (2014) Comparison of nonlinear optical chromophores containing different conjugated electron-bridges: the relationship between molecular structure properties and macroscopic electro-optic activities of materials. RSC Adv. doi:10.1039/c4ra09368b
Dhanya R, Kishore VC, Sudha Kartha C, Sreekumar K, Rani J (2008) Ground state and excited state dipole moments of alkyl substituted para-nitroaniline derivatives. Spectrochim Acta A 71:1355–1359
Mallakpour S, Zadehnazari A (2011) Advances in synthetic optically active condensation polymers. Express Polym Lett 5:142–181
Zadrozna I, Kaczorowska E (2008) Relationship between structure and nonlinear optical properties of new bisazo chromophores. Theoretical and experimental study. Struct Chem 19:131–135
Luo J, Qin J, Kang H, Ye C (2000) Synthesis and characterization of accordion main-chain azo-dye polymers for second-order optical non-linearity. Polym Int 49:1302–1307
Monnereau C, Blart E, Illien B, Paris M, Odobel F (2005) Study of the cross-linking mechanism of a copolymer containing an electrooptic chromophore. J Phys Org Chem 18:1050–1058
Verbiest T, Houbrechts S, Kauranen M, Clays K, Persoons A (1997) Second-order nonlinear optical materials: recent advances in chromophore design. J Mater Chem 7:2175–2189
Hashimoto H, Nakashima T, Hattori K, Yamada T, Mizoguchi T, Koyama Y, Kobayashi T (1999) Structures and non-linear optical properties of polar carotenoid analogues. Pure Appl Chem 71:2225–2236
Bouchouit K, Derkowska B, Migalska-Zalas A, Abede S, Benali-cherif N, Sahraou B (2010) Nonlinear optical properties of selected natural pigments extracted from spinach: carotenoids. Dyes Pigments 86:161–165
El Kouari Y, Migalska-Zalas A, Arof AK, Sahraoui B (2014) Computations of absorption spectra and nonlinear optical properties of molecules based on anthocyanidin structure. Opt Quant Electron. doi:10.1007/s11082-014-9965-4
Roshal AD, Mitina VG, Orlov VD, Ponomarev OA, Sukhorukov AA, Fialkova SV (1997) Interpretation of electron transitions in absorption spectra of phenylchromones and their hydroxy derivatives. Funct Mater 4:121–127
Doroshenko AO (1999) Spectral Data Lab Software. Institute of Chemistry at V.N. Karazin Kharkiv National University, Kharkiv
General methods of test for pigments and extenders. Part 10. Determination of density. Pyknometer method. ISO 787-10:1993
Lee H, Neville K (1967) Handbook of epoxy resins. McGraw-Hill, New York
Labanowski JK (1991) In: Andzelm JW (ed) Density functional methods in chemistry. Springer, New York
Zhao Y, Truhlar DG (2006) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA (1982) Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J Chem Phys 77:3654–3665
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theor Chim Acta 28:213–222
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC et al (2004) Gaussian 09, revision C.02; Gaussian, Inc. Wallingford
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094
Barone V, Cossi M, Mennucci B, Tomasi JA (1997) New definition of cavities for the computation of solvation free energies by the polarizable continuum model. J Chem Phys 107:3210–3221
Patterson WA (1954) Infrared absorption bands characteristic of the oxirane ring. Anal Chem 26:823–835
Bomstein J (1958) Infrared spectra of oxirane compounds. Correlations with structure. Anal Chem 30:544–546
González M, Cabanelas JC, Baselga J (2012) Applications of FTIR on epoxy resins—identification, monitoring the curing process, phase separation and water uptake. In Theophile T (ed) Infrared spectroscopy—materials science, engineering and technology. InTech Europe. doi:10.5772/36323
Hong Y-S, Hong KS, Cho J-H, Volkov VI, Lee C-H (2008) Chemical exchange of hydroxyl protons in quercetin measured by pulsed field gradient NMR. Appl Magn Reson 35:261–270
Mishina EN, Goloveshkin AC, Bushmarinov IS, Teleshev AT, Mishina VYu, Nifantiev EYe (2012) Structural peculiarities of 3,5,7,3′,4′-pentahydroxyflavone obtained from wood of the larch. Khim Rast Syrja 4:85–90
ACD/CNMR (1994–1996) Advanced chemistry development Inc., Toronto
Mattarei A, Biasutto L, Rastrelli F, Garbisa S, Marotta E, Zoratti M, Paradisi C (2010) Regioselective o-derivatization of quercetin via ester intermediates. An improved synthesis of rhamnetin and development of a new mitochondriotropic derivative. Molecules 15:4722–4736
Roshal AD, Sakhno TV, Verezubova AA, Ptiagina LM, Musatov VI, Wróblewska A, Błażejowski J (2003) Structure, stability and spectral properties of complexes of flavones with metal ions of group II. Funct Mater 10:419–426
Mezzetti A, Protti S, Lapouge Ch, Cornard J-P (2011) Protic equilibria as the key factor of quercetin emission in solution. Relevance to biochemical and analytical studies. Phys Chem Chem Phys 13:6858–6864
Musialik M, Kuzmicz R, Pawłowski TS, Litwinienko G (2009) Acidity of hydroxyl groups: an overlooked influence on antiradical properties of flavonoids. J Org Chem 74:2699–2709
Momić T, Savić J, Černigoj U, Trebše P, Vasić V (2007) Protolytic equilibria and photodegradation of quercetin in aqueous solution. Collect Czech Chem Commun 72:1447–1460
Agrawal PK, Schneider H-J (1983) Deprotonation induced 13C NMR shifts in phenols and flavonoids. Tetrahedron Lett 24:177–180
Reichardt C, Welton T (2011) Solvents and solvent effects in organic chemistry. Wiley-VCH, Weinheim
Paley MS, Harris JM, Looser H, Baumert JC, Bjorklund GC, Jundt D, Twieg RJ (1989) A solvatochromic method for determining second-order polarizabilities of organic molecules. J Org Chem 54:3774–3778
Mishurov D, Roshal A, Brovko O (2015) Second-order polarizability and temporal stability of epoxy polymers doped with chromophore and with chromophore moieties in the main chain. Polym Polym Compos 23:121–128
Acknowledgments
The authors gratefully acknowledge Professor J. Błażejowski and Professor K. Krzymiński from University of Gdansk for assistance with testing the structures of the glycidyl ethers. The Ukrainian-American Laboratory of Computational Chemistry (UALCC, Kharkiv, Ukraine) is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishurov, D.A., Voronkin, A.A. & Roshal, A.D. Synthesis, molecular structure and optical properties of glycidyl derivatives of quercetin. Struct Chem 27, 285–294 (2016). https://doi.org/10.1007/s11224-015-0694-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0694-5