Abstract
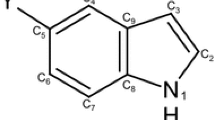
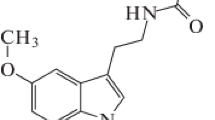
Theoretical calculations performed on the methoxyindole isomers using the B3LYP, MP2, and MP4 methods combined with the 6-311++G(d,p) and 6-311++G(3df,3pd) basis sets reveal that the preferred conformation exhibited by all isomers has the exocyclic group co-planar to the ring plane. Moreover, certain positions of the substituent are found to be far more stable than others. In order to rationalize these results, the harmonic oscillator model of aromaticity (HOMA), together with the natural bond orbital (NBO) and natural resonance theories (NRT), have been employed to evaluate the π-electron delocalization in the different molecules. To act as a reference, the study has been extended to indole. The donor–acceptor interactions were energetically quantified by using the NBO deletion method. In general, the results given by the three approaches are in good agreement and provide complementary data about the main effects of the position/orientation of the methoxy group on the electronic structure of the indole ring. The electron redistribution resulting from the H/OCH3 substitution was also analyzed in terms of the natural hybrid compositions of the πCC orbitals given by the NBO theory and atomic charges.





Similar content being viewed by others
References
Sunberg RJ (1996) Indoles. Academic Press, New York
Sundberg RJ (1970) The chemistry of indoles. Organic chemistry—a series of monographs, vol 18. Academic Press, New York
Joule John A, Mills K (2012) Heterocyclic chemistry at a glance, 2nd edn. Wiley, Chichester
Gribble GW (ed) (2010) Heterocyclic scaffolds II: reactions and applications of indoles. Topics in heterocyclic chemistry, vol 2. Springer, London
Sharma V, Kumar P, Pathak D (2010) J Heterocyclic Chem 47(3):491–502
Van Order RB, Lindwall HG (1942) Chem Rev 30(1):69–96
Huether G, Kochen W, Simat TJ, Steinhart S (eds) (1999) Tryptophan, serotonin, and melatonin: basic aspects and applications. Advances in experimental medicine and biology, vol. 467. Kluwer Academic, New York
Kaushik N, Kaushik N, Attri P, Kumar N, Kim C, Verma A, Choi E (2013) Molecules 18(6):6620–6662
Kochanowska-Karamyan AJ, Hamann MT (2010) Chem Rev 110(8):4489–4497
Mahboobi S, Pongratz H, Hufsky H, Hockemeyer J, Frieser M, Lyssenko A, Paper DH, Bürgermeister J, Böhmer F-D, Fiebig H-H, Burger NM, Baasner S, Beckers T (2001) J Med Chem 44(26):4535–4553
Zhang F, Zhao Y, Sun L, Ding L, Gu Y, Gong P (2011) Eur J Med Chem 46(7):3149–3157
Biswal S, Sahoo U, Sethy S, Kumar HKS, Banerjee M, Banerjee M (2012) Asian J Pharm Clin Res 5:1–6
Tarzia G, Diamantini G, Di Giacomo B, Spadoni G, Esposti D, Nonno R, Lucini V, Pannacci M, Fraschini F, Stankov BM (1997) J Med Chem 40(13):2003–2010
Bowden K, Grubbs EJ (1996) Chem Soc Rev 25(3):171–177
Taft RW, Lewis IC (1958) J Am Chem Soc 80(10):2436–2443
Campanelli AR, Domenicano A, Ramondo F (2003) J Phys Chem A 107(33):6429–6440
Kim CK, Han IS, Ryu WS, Lee HW, Lee B-S, Kim CK (2006) J Phys Chem A 110(7):2500–2504
Krygowski TM, Ejsmont K, Stepień BT, Cyrański MK, Poater J, Solà M (2004) J Org Chem 69(20):6634–6640
Krygowski TM, Stepień BT (2004) Pol J Chem 78:2213–2217
Krygowski TM, Stepień BT (2005) Chem Rev 105(10):3482–3512
Krygowski TM, Dobrowolski MA, Zborowski K, Cyrański MK (2006) J Phys Org Chem 19(12):889–895
Krygowski TM, Palusiak M, Płonka A, Zachara-Horeglad JE (2007) J Phys Org Chem 20(5):297–306
Mohajeri A, Shahamirian M (2010) J Mol Struct (Theochem) 951(1–3):72–76
Mohajeri A, Shahamirian M (2010) J Phys Org Chem 23(5):440–450
Becke AD (1988) Phys Rev A 38(6):3098–3100
Becke AD (1993) J Chem Phys 98(7):5648–5652
Lee CT, Yang WT, Parr RG (1988) Phys Rev B 37(2):785–789
Frisch MJ, Head-Gordon M, Pople JA (1990) Chem Phys Lett 166(3):275–280
Frisch MJ, Head-Gordon M, Pople JA (1990) Chem Phys Lett 166(3):281–289
Head-Gordon M, Head-Gordon T (1994) Chem Phys Lett 220(1–2):122–128
Krishnan R, Pople JA (1978) Int J Quantum Chem 14(1):91–100
Frisch GWT MJ, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J A Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) GAUSSIAN 09. A.02 edn. Gaussian, Inc., Wallingford
Weinhold F, Landis CR (2005) Valency and bonding: a natural bond orbital donor–acceptor perspective. Cambridge University Press, New York
Weinhold F, Landis CR (2012) Discovering chemistry with natural bond orbitals. Wiley, Hoboken
Glendening ED, Landis CR, Weinhold F (2012) WIREs Comput Mol Sci 2(1):1–42
Glendening ED, Badenhoop JK, Weinhold F (1998) J Comput Chem 19(6):628–646
Glendening ED, Weinhold F (1998) J Comput Chem 19(6):593–609
Glendening ED, Weinhold F (1998) J Comput Chem 19(6):610–627
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2001) NBO 5.G. Theoretical Chemistry Institute, University of Wisconsin, Madison
Krygowski TM, Cyrański MK (2001) Chem Rev 101(5):1385–1420
Krygowski TM, Szatylowicz H, Stasyuk OA, Dominikowska J, Palusiak M (2014) Chem Rev 114(12):6383–6422
Balaban AT, Oniciu DC, Katritzky AR (2004) Chem Rev 104(5):2777–2812
Cyrañski MK, Ksawery M (2005) Chem Rev 105(10):3773–3811
Krygowski TM (1993) J Chem Inf Comput Sci 33(1):70–78
Kruszewski J, Krygowski TM (1972) Tetrahedron Lett 13(36):3839–3842
Katritzky AR, Jug K, Oniciu DC (2001) Chem Rev 101(5):1421–1450
Madura ID, Krygowski TM, Cyrañski MK (1998) Tetrahedron 54(49):14913–14918
Andrzejak M, Kubisiak P, Zborowski K (2013) Struct Chem 24(4):1171–1184
Raczyńska ED, Hallman M, Kolczyńska K, Stępniewski TM (2010) Symmetry 2(3):1485–1509
Bansal RK (1999) Heterocyclic chemistry, 3rd edn. New Age International Publishers, New Delhi
Lopes Jesus AJ, Redinha JS (2013) Comput Theor Chem 1023(0):74–82
Breneman CM, Wiberg KB (1990) J Comput Chem 11(3):361–373
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lopes Jesus, A.J., Redinha, J.S. Methoxyindoles: stability and π-electron delocalization. Struct Chem 26, 655–666 (2015). https://doi.org/10.1007/s11224-014-0520-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0520-5