Abstract
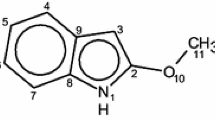
A detailed energetic and structural analysis of indole derivatives is performed by combining the results of B3LYP calculations with the data provided from the application of the natural bond orbital (NBO) theory. The following electron-donating and electron-accepting substituents have been considered: NO, NO2, CN, CH3, F, OCH3, OH and NH2. By using a homodesmic reaction, the substituent effect stabilization energies are evaluated. It is shown that the electron-donating and electron-accepting groups originate opposite effects when they interact with the indole ring. Attention is also given to the relative stabilization of the different substitution sites and of the different orientations of the substituents. In order to shed light into the origin of the global energetic effects of the indole substitution, the deletion and second-order perturbation methods implemented in the NBO analysis are applied. Special emphasis is paid to the effect of the endo- and exocyclic π-delocalizations. Connecting certain substituents to specific carbon centers leads to the formation of intramolecular H-bonds, which are here characterized by using geometrical and electronic descriptors. Their impact on the molecular stability and their interplay with the π-electron delocalization is investigated.






Similar content being viewed by others
References
Ramesh Dhani A, Avinash SK, Salenaagina MV, Saicharan Teja P, Masthanaiah Rathnam PR, Silpa VC (2011) J Chem Pharm Res 3(5):519–523
Sharma V, Kumar P, Pathak D (2010) J Heterocyclic Chem 47(3):491–502
Sunberg RJ (1996) Indoles. Academic Press, New York
Gribble GW (ed) (2010) Heterocyclic scaffolds II: reactions and applications of indoles, topics in heterocyclic chemistry, vol 2. Springer, London
Parry RJ (2008) Biosynthesis of compounds containing an indole nucleus. In: Chemistry of heterocyclic compounds. Wiley, pp 1–64
Barden T (2010) Indoles: industrial, agricultural and over-the-counter uses. In: Gribble GW (ed) heterocyclic scaffolds II, vol 26., Topics in heterocyclic chemistrySpringer, Berlin, pp 31–46
Kaushik N, Kaushik N, Attri P, Kumar N, Kim C, Verma A, Choi E (2013) Molecules 18(6):6620–6662
Biswal S, Sahoo U, Sethy S, Kumar HKS, Banerjee M (2012) Asian J Pharm. Clin Res 5:1–6
Welsch ME, Snyder SA, Stockwell BR (2010) Curr Opin Chem Biol 14(3):347–361
Johansson H, Jorgensen TB, Gloriam DE, Brauner-Osborne H, Pedersen DS (2013) RSC Adv 3(3):945–960
Krygowski TM, Ejsmont K, Stepień BT, Cyrański MK, Poater J, Solà M (2004) J Org Chem 69(20):6634–6640
Krygowski TM, Dobrowolski MA, Zborowski K, Cyrański MK (2006) J Phys Org Chem 19(12):889–895
Krygowski TM, Stepień BT (2004) Pol J Chem 78:2213–2217
Cyrañski MK, Ksawery M (2005) Chem Rev 105(10):3773–3811
Mohajeri A, Shahamirian M (2010) J Mol Struct (Theochem) 951(1–3):72–76
Alagona G, Ghio C, Monti S (1998) J Mol Struct (Theochem) 433(1–3):203–216
Kettle LJ, Bates SP, Mount AR (2000) PCCP 2(2):195–201
Lopes Jesus AJ, Redinha JS (2014) Struct Chem. doi:10.1007/s11224-014-0520-5:1-12
Perrin L, Andre F, Aninat C, Ricoux R, Mahy J-P, Shangguan N, Joullie MM, Delaforge M (2009) Metallomics 1(2):148–156
Becke AD (1988) Phys Rev A 38(6):3098–3100
Becke AD (1993) J Chem Phys 98(7):5648–5652
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72(1):650–654
Krygowski TM, Szatylowicz H, Stasyuk OA, Dominikowska J, Palusiak M (2014) Chem Rev 114(12):6383–6422
Siodła T, Ozimiński WP, Hoffmann M, Koroniak H, Krygowski TM (2014) J Org Chem 79(16):7321–7331
Palusiak M, Domagala M, Dominikowska J, Bickelhaupt FM (2014) PCCP 16(10):4752–4763
Mohajeri A, Shahamirian M (2010) J Phys Org Chem 23(5):440–450
Singla N, Bhadram VS, Narayana C, Chowdhury P (2013) J Phys Chem A 117(13):2738–2752
Krygowski TM, Palusiak M, Płonka A, Zachara-Horeglad JE (2007) J Phys Org Chem 20(5):297–306
Frisch MJ, Head-Gordon M, Pople JA (1990) Chem Phys Lett 166(3):281–289
Frisch MJ, Head-Gordon M, Pople JA (1990) Chem Phys Lett 166(3):275–280
Head-Gordon M, Head-Gordon T (1994) Chem Phys Lett 220(1–2):122–128
M. J. Frisch GWT, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox (2009) GAUSSIAN 09. A.02 edn. Gaussian, Inc., Wallingford CT
Weinhold F, Landis CR (2005) Valency and bonding: A Natural Bond Orbital Donor-Acceptor Perspective. Cambridge University Press, New York
Weinhold F, Landis CR (2012) Discovering chemistry with natural bond orbitals. Wiley, New Jersey
Glendening ED, Landis CR, Weinhold F (2012) WIREs Comput Mol Sci 2(1):1–42
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2001) NBO 5.0 Theoretical Chemistry Institute, University of Wisconsin, Madison
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Bader RFW (1990) Atoms in molecules: a quantum theory. University Press, Oxford
Bader RFW (1991) Chem Rev 91(5):893–928
Biegler-König F, Schönbohm J, Bayles D (2001) J Comput Chem 22(5):545–559
Exner O, Bohm S (2000) J Chem Soc Perkin Trans 2(9):1994–2000
Krygowski TM, Stepień BT (2005) Chem Rev 105(10):3482–3512
Charton M (2007) Electrical effect substituent constants for correlation analysis. In: Progress in physical organic chemistry. Wiley, pp 119–251
Pross A, Radom L, Taft RW (1980) J Org Chem 45(5):818–826
Hammett LP (1937) J Am Chem Soc 59(1):96–103
Hansch C, Leo A, Taft RW (1991) Chem Rev 91(2):165–195
Domingo LR, Pérez P, Contreras R (2003) J Org Chem 68(15):6060–6062
Arunan E, Desiraju GR, Klein RA, Sadlej J, Scheiner S, Alkorta I, Clary DC, Crabtree RH, Dannenberg JJ, Hobza P, Kjaergaard HG, Legon AC, Mennucci B, Nesbitt DJ (2011) Pure Appl Chem 83:1637–1641
Grabowski SJ (ed) (2006) Hydrogen bonding—new insights (Challenges and advances in computational chemistry and physics). Springer, Dordrecht
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, Oxford
Jeffrey GA, Saenger W (1991) Hydrogen bonding in biological structures. Springer, New York
Steiner T (2002) Angew Chem Int Edit 41(1):48–76
Koch U, Popelier PLA (1995) J Phys Chem 99(24):9747–9754
Desiraju GR, Steiner T (1999) The weak hydrogen bondin structural chemistry and biology. Oxford University Press, New York
Pacios LF, Gómez PC (2001) J Comput Chem 22(7):702–716
Espinosa E, Molins E, Lecomte C (1998) Chem Phys Lett 285(3–4):170–173
Gilli G, Gilli P (2009) The nature of the hydrogen bond: outline of a comprehensive hydrogen bond theory. Oxford University Press, New York
Gilli P, Bertolasi V, Ferretti V, Gilli G (1994) J Am Chem Soc 116(3):909–915
Gilli G, Bellucci F, Ferretti V, Bertolasi V (1989) J Am Chem Soc 111(3):1023–1028
Sobczyk L, Grabowski SJ, Krygowski TM (2005) Chem Rev 105(10):3513–3560
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lopes Jesus, A.J., Redinha, J.S. Energetic and electronic study of indole derivatives. Struct Chem 27, 809–820 (2016). https://doi.org/10.1007/s11224-015-0635-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0635-3