Abstract
Quantum chemistry studies of various tautomeric/rotameric forms of the furanyl analogues of acetyl dihydroxychalcone (FC) and dihydroxydichalcone (FDC) have shown that the thermodynamically most stable molecules are planar. In the crystalline solid phase, both molecules are more (FDC) or less (FC) non-planar. Two relatively strong intramolecular H-bonds, whose existence is evidenced by X-ray, computational, and spectral investigations, stabilize the planar geometry and influence the features of the lowest energy tautomers/rotamers of both compounds. Extending to the visible region, the electronic absorption of FC and FDC is accompanied by an intramolecular electron density shift, a property that could have analytical implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Derivatives of (2E)-1,3-diphenylprop-2-en-1-one (chalcones) are naturally occurring compounds which, due to their biological activity, have been the subject of interest to the food and health sciences [1, 2]. They have been thoroughly investigated in the context of their role in plant growth, human diet, and healthcare [1–7]. Our interest in these compounds arises from the fact that they are precursors in the synthesis of flavonols, which are derivatives of 3-hydroxy-2-phenyl-4H-chromene-4-one [8–10]. Following the synthesis of various chalcones (C) and dichalcones (DC) [derived from (2E,2′E)-1,1′-(1,3-phenylene)bis(3-phenylprop-2-en-1-one)] and related derivatives, we obtained two compounds that were furanyl (F) analogues of these entities, namely, 1-(5-acetyl-2,4-dihydroxyphenyl)-3-(furan-2-yl)prop-2-en-1-one (FC) and 1,1′-(4,6-dihydroxybenzene-1,3-diyl)bis[3-(furan-2-yl)prop-2-en-1-one] (FDC). The molecules of these compounds provide convenient models for studying the properties of intramolecular H-bonds, as there are two sites present in them where a hydroxyl group appears in the vicinity of the carbonyl group and where such bonds can occur. Another interesting property of FC and FDC is that both can exist in several tautomeric and rotameric forms. With these compounds in hand, we can investigate these phenomena. Finally, we may infer from the canonical structures of FC and FDC that both molecules should be planar. Thus, it would be interesting to know how such relatively large molecules are packed in the crystalline solid phase. To shed more light on these issues and to elucidate the properties of both these molecules, we carried out X-ray, spectroscopic, and computational investigations, the results of which are presented in this publication. A search of the Cambridge Structure Database [11] showed that the structures of several chalcones have been determined by X-ray diffractometry, but that no structures of dichalcones have as yet been established.
Materials and methods
Synthesis and spectral investigations
1-(5-Acetyl-2,4-dihydroxyphenyl)-3-(furan-2-yl)prop-2-en-1-one (FC) and 1,1′-(4,6-dihydroxybenzene-1,3-diyl)bis[3-(furan-2-yl)prop-2-en-1-one] (FDC) were synthesized by condensation (with removal of H2O) of commercially available 1,1′-(4,6-dihydroxybenzene-1,3-diyl)diethanone with furan-2-carbaldehyde dissolved in methanol/50 % aqueous NaOH (1/1 v/v) [8, 9, 12]. The reactant mixture was kept at room temperature for 48 h, then diluted twice with distilled water and neutralized with aqueous HCl to pH 5. The resulting precipitate was filtered off, rinsed with hot (333–343 K) water and dried. The products obtained were separated by gravitational column chromatography (Silica Gel 60, ethyl acetate/hexane, 1/2 v/v). Yellow-orange and orange crystals of FC and FDC, respectively, suitable for X-ray and other investigations were grown from an ethanol/n-hexane (4/1 v/v) solution (FC: m.p. 452–453 K, m/z = 273 (M–H+); FDC: m.p. 444–445 K, m/z = 351 (M–H+)).
The relevant spectral grade solvents were used throughout in the spectroscopic investigations. UV spectra were recorded on a Hitachi U3210 spectrophotometer. IR spectra were recorded on a Bruker FTIR IFS 66 spectrophotometer and NMR spectra on a Varian Unity 500 Plus spectrometer operating at a proton frequency of 500 MHz (carbon frequency = 125 MHz).
Single crystal X-ray measurements
X-ray data for the title compounds were collected on an Oxford Diffraction Gemini R Ultra Ruby CCD diffractometer (MoKα radiation, graphite monochromator). Data collection and cell refinement were carried out with CrysAlis CCD and data reduction with CrysAlis RED. Structure solution and refinement was carried out with the SHELXS97 package. All C and O atoms were refined anisotropically. H atoms of C–H bonds were positioned geometrically and constrained to ride on their parent atoms, H atoms involved in O–H···O hydrogen bonds were located on a difference Fourier map and refined isotropically. The asymmetric unit consists of two crystallographically independent molecules of FC and one half of the molecule of FDC.
Computations
Geometry optimizations were carried out at the DFT level of theory [13] with the B3LYP functional [14–17] and 6–31G** basis set [18, 19] and, in the case of selected entities, at the MP2 level [20] using the cc-pVDZ basis set [21, 22] and the Gaussian 03 program package [23].
Results and discussion
According to the relevant canonical structures (Figs. 1 and 1S, 2S in the Supplementary data), we can expect FC and FDC to have, respectively, five and three tautomeric forms (resulting from the migration of the H atom between two O atoms) with two or one intramolecular H-bond(s) [24]. In the case of only one intramolecular H-bond, four tautomeric/rotameric canonical structures can be expected for both FC and FDC. Rotation of furanyl fragment(s) around a C–C bond, examined to ascertain whether the crystalline phase consists of the lowest energy rotamers, makes it possible for each FC tautomer to occur in two rotameric forms, and each FDC tautomer in three or four such forms. Investigation of the rotation of the O–H group around a C–O bond in four tautomers/rotamers of both FC and FDC enabled us to ascertain whether this would be an adequate reflection of the thermodynamics of H-bonds. Tautomerism may also result from the migration of the H atom between C and O, O and C, or two O atoms [24], which leads to a substantial increase in the number of tautomers. To take into account such a possibility we considered selected structures relevant to the a1 forms (found to be the lowest energy ones) of FC (fifteen structures) and FDC (four structures) (Fig. 3S in the Supplementary data).
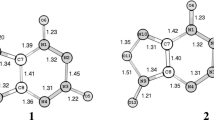
Optimization of the canonical structures a1, b1, and c1 of FC or a1 and b1 of FDC leads to the most stable molecular structure a1 for each compound (Fig. 2). An analogous result was obtained for the respective rotamers of FC (a2) and FDC (a2 and a3); the relevant molecular structures are shown in Figs. 4S–6S (Supplementary data), also those in which only one H-bond is present (d and e of FC, and c of FDC). The data in Table 1 (Tables 1S, 2S in the Supplementary data) indicate that the thermodynamically most stable tautomer of FC and FDC is predicted to be a, which can occur as two rotamers (a1 and a2) in the former compound and three rotamers (a1, a2, and a3) in the latter. The enthalpy and Gibbs free energy differences between the most and the least stable rotamers of a are no greater than a dozen or so kJ/mol, which implies that various entities of this kind can occur in equilibrium at room temperature (the equilibrium constants expressing the ratio of the number of molecules of a2 and a1 should be 0.201 and 0.152 for FC and FDC, respectively; from MP2 data). Other tautomers/rotamers of both compounds, including those resulting from the migration of the H atom between C–O, O–C, or two O atoms are over 89 kJ/mol thermodynamically less stable than tautomer/rotamer a1 and cannot be expected to be present in measurable amounts under such conditions.
MP2 optimized structures of selected tautomeric forms of FC and FDC (for canonical structures, see Fig. 1)
The stability of tautomers a [b, c, a1(i)–a1(vii)] of FC and a (b) of FDC should be substantially enhanced by two intramolecular H-bonds (Figs. 1 and 1S–3S in the Supplementary data). In other tautomers of the compounds considered only one such bond occurs. Therefore, the differences between the enthalpy (ΔH) and Gibbs free energy (ΔG) of the respective tautomeric/rotameric forms [d, e and a (b, c) of FC and c and a (b) of FDC] could tentatively be regarded as relevant thermodynamic characteristics of a single H-bond (tautomeric forms resulting from the migration of the H atom between C and O, O and C or two O atoms cannot be used for such estimations). The ΔH and ΔG values shown in Tables 1 and 2S (Supplementary data) are, however, much higher than those we found earlier for a single H-bond (ΔG for such a bond was estimated to be 78.3 kJ/mol [25]). Presumably, then, the transformation from tautomer a to other tautomers of FC (d, e) and FDC (c) is accompanied not only by the disappearance of one H-bond, but also by substantial structural changes (Figs. 2 and 4S–6S in the Supplementary data), which lead to the additional thermodynamic destabilization of the latter molecules. If we compare tautomer a with tautomers d and e of FC, and tautomer a with tautomer c of FDC, we find that they occur as a result of H atom transfer between non-vicinal sites of the molecules. On the other hand, the forms b* and c* originating from b and c of FC and b* originating from b of FDC, which occur as a result of H atom transfer between vicinal sites of the molecules, are thermodynamically much less stable. The ΔH and ΔG values of the respective latter forms are comparable to the former ones mentioned above, which means that they probably do not reflect thermodynamic characteristics of the single H-bond. The forms b* and c* of FC and b* of FDC can exist since there is an enthalpy and Gibbs free energy barrier to a H atom returning to the position it occupied in tautomers b and c of FC and b of FDC, respectively (the structures of molecules in transition states for such a process are presented in Figs. 7S and 8S, and the activation barriers are given in Table 3S in the Supplementary data).
Crystals of FC and FDC consist of tautomers/rotamers a1, as demonstrated by the results of X-ray investigations [the details of the refinement (Tables 4S, 5S) are given in the Supplementary data]. Two structurally similar but crystallographically independent molecules A and B are present in the asymmetric unit of FC (Figs. 3 and 9S in the Supplementary data), while one half of the molecule of C 2 symmetry is present in the asymmetric unit of FDC (Figs. 4 and 10S in the Supplementary data). The molecular structure of FC is stabilized by two S(6) type intramolecular O–H···O H-bonds (Table 6S in the Supplementary data), and the crystal structure of the compound by a network of intermolecular C–H···O interactions (Table 7S and Fig. 11S in the Supplementary data). The molecular structure of FDC is also stabilized by two S(6) type intramolecular O–H···O H-bonds (Table 8S in the Supplementary data). In the crystal structure, the parallel FDC molecules interacting via C=O···π contacts (Table 9S in the Supplementary data) are arranged in columns along the a axis; the inverted columns in which molecules are in contact via C–H···O interactions (Table 10S in the Supplementary data) form pairs of columns, each of which interacts dispersively with four adjacent pairs of columns stabilizing the crystal lattice (Figs. 12S, 13S in the Supplementary data).
The experimentally determined structural parameters of the a1 forms of the compounds investigated compared well with the predicted values (Tables 2, 3) according to the criteria we applied earlier [25]. The lowest energy forms (a1) of both FC and FDC (C 2v symmetry) should be planar in the gaseous phase (Table 4). In the crystalline solid phase both compounds are less (FC) and more (FDC) non-planar. Other tautomers/rotamers of FC and FDC are predicted to be either planar (a2 and c1* of FC, and a2 and a3 of FDC) or non-planar (Tables 4 and 11S in the Supplementary data). Low energy rotamers resulting from the rotation of furanyl fragment(s) around a C–C bond (a1 and a2 of FC, and a1, a2 and a3 of FDC) are predicted to be planar, whereas rotamers arising from the rotation of the OH group around a C–O bond (b1*, b2* and c2* of FC, and b1*, b2*, b3*, and b4* of FDC) ought to be non-planar. It is thus evident that the disappearance of one of the H-bonds in the latter rotamers is accompanied by a loss of planarity. Another reason for the loss of planarity is the partial disappearance of resonance conjugation from the molecular systems of FC [in tautomers a1(vi), a1(vii), a1(x), a1(xi), a1(xiv), and a1(xv)] and FDC [a1(iii) and a1(iv)] (Fig. 3S in the Supplementary data). The thermodynamic measure of this effect can be given by the difference in Gibbs free energies of the relevant tautomers of the series a1(i)–a1(xv) of FC and a1(i)–a1(iv) of FDC. The lowest such differences [i.e. relevant to a1(x)–a1(viii) and a1(xv)–a1(xiii) of FC, and a1(iii)–a1(ii) of FDC], ranging from 24 to 32 kJ/mol, reflect the extent of the thermodynamic destabilization of both molecules caused by the partial disappearance of conjugation in them.
C=O bonds in molecules in which O atoms are involved in (C)O···H–O interactions, whose geometry is described in Tables 6S and 8S (Supplementary data) and demonstrated in Figs. 2 and 4S–6S (Supplementary data), are usually longer than those not engaged in such interactions (Tables 4 and 11S in the Supplementary data). On the other hand, the C–O bond length, if the O atom is a part of the hydroxyl group, is not affected by the involvement or non-involvement of this group in H-bonding interactions. The involvement of the above mentioned fragments of FC and FDC molecules in intramolecular O–H···O bonds is confirmed by IR and NMR spectral data: the wavenumbers of the C=O and O–H stretching vibrations determined experimentally agree qualitatively with the predicted values (Table 5) [25, 26], and the 1H signals of H atoms from hydroxyl groups engaged in such interactions appear at relatively high chemical shifts (Table 6) [25, 27].
Extension of the electronic absorption of FC and FDC to the visible region (Figs. 5, 6) confirms that both molecular systems are conjugated and planar. The long wavelength absorption accompanied by an electron density shift from 4,6-dihydroxy-1,3-phenylene (in both FC and FDC) to the one (FC) or two (FDC) (1E)-1-(furan-2-yl)-3-oxoprop-1-en-3-yl fragments, as reflected by changes in the sum of the atomic partial charges of these fragments (Table 7) and the contours of the HOMO and LUMO orbitals of molecules in the ground and electronically excited states (Figs. 14S, 15S in the Supplementary data), is similar in both compounds (falling within the 370–380 nm range). This implies that the extent of conjugation does not affect the spectral behaviour of FDC. Electronic excitation is also accompanied by loss of planarity in both FC and FDC (Fig. 16S in the Supplementary data). Such substantial structural and electron density changes accompanying electronic excitation make these compounds potential absorption spectral indicators for physicochemical and analytical investigations. On the other hand, the compounds investigated do not luminesce, so they cannot be used as fluorescent probes.
The UV–vis absorption (solid line) and deconvoluted (using Spectral Data Lab software [35]) (dashed lines) spectra of FC (dissolved in methanol) together with predicted (at the TDDFT(B3LYP)/6–31G** level of theory for structure a1) electronic absorption transitions (bars)
The UV–VIS absorption (solid line) and deconvoluted (using Spectral Data Lab software [35]) (dashed lines) spectra of FDC (dissolved in methanol) together with predicted (at the TDDFT(B3LYP)/6-31G** level of theory for structure a1) electronic absorption transitions (bars)

Conclusions
To summarize, only one tautomeric/rotameric form of FC and FDC has been found in the crystalline solid phase, which corresponds to the lowest energy form of the isolated molecules. Two intramolecular H-bonds influencing the crystal structures of FC and FDC also occur in the liquid phase and affect the spectral properties of both molecules. The quite large FC and FDC molecules, predicted to be planar in the ground state become non-planar in the electronically excited state. Electronic excitation, accompanied by a substantial interfragmental shift of electron density, makes these compounds potential spectral indicators for physicochemical and analytical applications.
Supplementary data
Complete X-ray data have been deposited with the Cambridge Crystallographic Data Centre (CCDC) as supplementary publications Nos. 854359 (FC) and 854360 (FDC); copies of these data can be obtained, free of charge, on application to CCDC, 12 Union Raod, Cambridge CB2 1EZ, UK (fax: +44-(0)1223-336033 or e-mail: deposit@ccdc.cam.ac.uk).
References
Grayer RJ, Veitch NC (2006) In: Andersen ØM, Markham KR (eds) Flavonoids: chemistry, biochemistry and applications. CRC Press/Taylor & Francis Group, Boca Raton
Grotewold E (2006) The science of flavonoids. Springer, New York
Downey MO, Harvey JS, Robinson SP (2003) Aust J Grape Wine Res 9:110–121
Pettit GR, Mukku VJRV, Gragg G, Herald DL, Knight JC, Herald CL, Chapuis J-C (2008) J Nat Prod 71:130–133
Wang L, Lee I-M, Zhang SM, Blumberg JB, Buring JE, Sesso HD (2009) Am J Clin Nutr 89:905–912
Combet E, Lean MEJ, Boyle JG, Crozier A, Davidson DF (2011) Clin Chim Acta 412:165–169
Britton RG, Horner-Glister E, Pomenya OA, Smith EE, Denton R, Jenkins PR, Steward WP, Brown K, Gescher A, Sale S (2012) Eur J Med Chem 54:952–958
Smith MA, Neumann RM, Webb RA (1968) J Heterocycl Chem 5:425–426
Aitmambetov A, Kubzheterova A (2002) Russ J Bioorg Chem 28:165–166
Chen P-Y, Wang T-P, Chiang MY, Huang K-S, Tzeng C-C, Chen Y-L, Wang E-C (2011) Tetrahedron 67:4155–4160
Allen FH, Kennard O, Taylor R (1983) Acc Chem Res 16:146–153
Wera M, Chalyi AG, Roshal AD, Błażejowski J (2012) Acta Crystallogr Sect E 68:o253–o254
Labanowski JK, Andzelm JW (1991) Density functional methods in chemistry. Springer, New York
Becke AD (1988) Phys Rev A 38:3098–3100
Becke AD (1993) J Chem Phys 98:1372–1377
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Hariharan PC, Pople JA (1973) Theor Chim Acta 28:213–222
Hehre WJ, Radom L, Schleyer PvR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Møller C, Plesset MS (1934) Phys Rev 46:618–622
Davidson E, Feller D (1986) Chem Rev 86:681–696
Dunning THJ (1989) Chem Phys 90:1007–1023
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision C.02. Gaussian, Inc., Wallingford, CT
Raczyńska ED, Kosińska W, Ośmiałowski B, Gawinecki R (2005) Chem Rev 105:3561–3612
Serdiuk IE, Wera M, Roshal AD, Sowiński P, Zadykowicz B, Błażejowski J (2011) Tetrahedron Lett 52:2737–2740
Emmeluth C, Suhm MA, Luckhaus DJ (2003) Chem Phys 118:2242–2255
Hansen PE, Bolvig S, Wozniak KJ (2005) J Mol Struct 749:155–168
Ditchfield R (1974) Mol Phys 27:789–807
Wolinski K, Hinton JF, Pulay P (1990) J Am Chem Soc 112:8251–8260
Gauss J (1993) J Chem Phys 99:3629–3643
Krzymiński K, Malecha P, Zadykowicz B, Wróblewska A, Błażejowski J (2011) Spectrochim Acta A 78:401–409
Mulliken RS (1955) J Chem Phys 23:1833–1840
Mulliken RS (1955) J Chem Phys 23:1841–1846
Besler BH, Merz KM, Kollman PA (1990) J Comput Chem 11:431–439
Spectral Data Lab© software (2003) Institute of Chemistry at Kharkiv National University, Kharkiv
Acknowledgments
This study was financed by the State Funds for Scientific Research (Grant DS/530-8220-D184-12) and from Grants Nos. 538-8221-1067-12 and 538-8220-B004-13. M.W. acknowledges financial support from the European Social Fund within the project “Educators for the elite—integrated training program for PhD students, Post-docs and Professors as academic teachers at the University of Gdańsk”, and the Human Capital Operational Programme Action 4.1.1, “Improving the quality on offer at tertiary educational institutions”. This publication reflects the views only of the authors: the sponsor cannot be held responsible for any use which may be made of the information contained herein. The calculations were carried out on computers of the Tri-City Academic Network Computer Centre in Gdańsk (TASK) and the Wroclaw Centre for Networking and Supercomputing (WCSS).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Wera, M., Chalyi, A.G., Roshal, A.D. et al. Structure, tautomerism, and features of 1-(5-acetyl-2,4-dihydroxyphenyl)-3-(furan-2-yl)prop-2-en-1-one (FC) and 1,1′-(4,6-dihydroxybenzene-1,3-diyl)bis[3-(furan-2-yl)prop-2-en-1-one] (FDC). Struct Chem 25, 969–977 (2014). https://doi.org/10.1007/s11224-013-0378-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-013-0378-y