Abstract
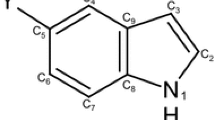
The global and local quantum chemical reactivity descriptors of a series of imidazole derivatives substituted at 2, 4, and 5 positions with different groups including electron-donating and electron-withdrawing substituents have been calculated using the B3LYP/6-311++G(d,p) and MP2/6-311++G(d,p) methods. The substituents have been selected to cover a wide range of electronic effects. Considering the calculated Fukui functions, both imidazole derivatives and their anions are found to be suitable nucleophilic sites in the gas phase. For the most substituents it was observed that the calculated Fukui function \(f_{\text{k}}^{ - }\) values at the N-site are small in case of electron-releasing substituents indicating a preferred N-site for hard reaction. In contrast, large \(f_{\text{k}}^{ - }\) values in case of electron-attracting groups indicate a preferred N-site for soft reaction. These two local descriptors predicted the reactivity of the electron-rich imidazole sequence to be 2-substituted imidazoles > 5-substituted imidazoles > 4-substituted imidazole where reactivity toward electrophilic attack at a pyridine nitrogen atom is enhanced by electron donor substituents elsewhere in the molecule, due to resonance effect.




Similar content being viewed by others
References
Tadokoro M, Nakasuji K (2000) Coord Chem Rev 198:205–218
Sunatsuki Y, Motoda Y, Matsumoto N (2002) Coord Chem Rev 226:205–209
Rowan R, Tallon T, Sheahan AM, Curran R, McCann M, Kavanagh K, Devereux M, McKee V (2006) Polyhedron 25:1771–1778
Fu YM, Zhao YH, Lan YQ, Wang Y, Qiu YQ, Shao KZ, Su ZM (2007) Inorg Chem Commun 10:720–723
Higa T, Moriya M, Shimazaki Y, Yajima T, Tani F, Karasawa S, Nakano M, Naruta Y, Yamauchi O (2007) Inorg Chem Acta 360:3304–3313
Deschamps P, Kulkarni PP, Gautam-Basak M, Sarkar B (2007) Coord Chem Rev 249:895–909
Rabenstein DL, Daignault SA, Isab AA, Arnold AP, Shoukry MM (1985) J Am Chem Soc 107:6435–6439
Varnagy K, Sovago I, Suli-Vargha H, Sanna D, Micera G (2000) J Inorg Biochem 81:35–41
Pogni R, Lunga GD, Basosi R (1993) J Am Chem Soc 115:1546–1550
Osz K, Varnagy K, Suli-Vargha H, Csampay A, Sanna D, Micera G, Sovago I (2004) J Inorg Biochem 98:24–32
Mendez F, Gazquez JL (1994) J Am Chem Soc 116:9298–9301
Li Y, Evans JNS (1995) J Am Chem Soc 117:7756–7759
Nguyen LT, Le TN, De Proft F, Chandra AK, Langenaeker W, Nguyen MT, Geerlings P (1999) J Am Chem Soc 121:5992–6001
Pal S, Chandrakumar KRS (2000) J Am Chem Soc 122:4145–4153
Perez P, Simon-Manso Y, Aizman A, Fuentealba P, Contreras R (2000) J Am Chem Soc 122:4756–4762
Geerlings P, De Proft F, Langenaeker W (2003) Chem Rev 103:1793–1873
Chattaraj PK, Sarkar U, Roy DR (2006) Chem Rev 106:2065–2091
Chattaraj PK, Maiti B, Sarkar U (2003) J Phys Chem A 107:4973–4975
Roy DR, Parthasarathi R, Padmanabhan J, Sarkar U, Subramanium V, Chattaraj PK (2006) J Phys Chem A 110:1084–1093
Padmanabhan J, Parthasarathi R, Elango M, Subramanian V, Krishnamoorthy BS, Gutierrez-Oliva S, Toro-Labbe A, Roy DR, Chattaraj PK (2007) J Phys Chem A 111:9130–9138
Jaramillo P, Perez P, Contreras R, Tiznado W, Fuentealba P (2006) J Phys Chem A 110:8181–8187
Jaramillo P, Domingo LR, Chamorro E, Perez P (2008) J Mol Struct (theochem) 865:68–72
Perez P, Domingo LR, Doque-Norena M, Chamorro E (2009) J Mol Struct (theochem) 895:86–91
Gazquez JL, Cedillo A, Vela A (2007) J Phys Chem A 111:1966–1970
Pratihar S, Roy S (2010) J Org Chem 75:4957–4963
Deuri S, Phukan P (2012) Comput Theor Chem 980:49–55
Deuri S, Phukan P (2010) J Mol Struct (Theochem) 945:64–70
Soleiman SM (2012) Comput Theor Chem 994:105–111
Pilepic V, Urslic S (2001) J Mol Struct (Theochem) 538:41–49
Richaud A, Mendeze F (2012) J Mex Chem Soc 56:351–354
Chermahini AN, Hosseinzadeh B, Beni AS, Teimouri A (2012) Comput Theor Chem (Theochem) 994:97–104
Chermahini AN, Ghaedi A, Teimouri A, Momenbeik F, Dabbagh HA (2008) J Mol Struct (theochem) 867:78–84
Chermahini AN, Teimouri A, Beni AS (2013) Comput Theor Chem (Theochem) 1008:67–73
Chermahini AN, Beni AS, Sharghi H (2011) J Korean Chem Soc 55:392–399
Dabbagh HA, Rasti E, Chermahini AN (2010) J Mol Struct (Theochem) 947:92–100
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega NA, Petersson G, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision B.01. Gaussian, Inc., Wallingford
Parr RG, Donnelly RA, Levy M, Palke WE (1978) J Chem Phys 68:3801–3808
Parr RG, Szentpaty LV, Liu S (1999) J Am Chem Soc 121:1922–1924
Yang W, Mortier WJ (1986) J Am Chem Soc 108:5708–5711
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Ozcan M, Dehri I, Erbil M (2004) Appl Surf Sci 236:155–164
Pearson RG (1963) J Am Chem Soc 85:3533–3543
Klopman G, Hudson RF (1967) Theor Chem Acta 8:165–174
Klopman G (1968) J Am Chem Soc 90:223–234
Chattaraj PK, Maiti B (2001) J Phys Chem A 105:169–183
Chermette H (1999) J Comput Chem 20:129–154
Roy RK, De Proft F, Geerlings P (1998) J Phys Chem A 102:7035–7040
Langenaeker W, De Proft F, Geerlings P (1996) J Mol Struct (theochem) 362:175–179
Arulmozhiraja S, Kolandaivel P (1997) Mol Phys 90:55–62
Langenaeker W, De Decker M, Geerlings P (1990) J Mol Struct (theochem) 207:115–130
Gazquez JL, Galvan M, Vela A (1990) J Mol Struct (theochem) 210:29–38
Chandra AK, Michalak A, Nguyen MT, Nalewajski RF (1998) J Phys Chem 102:10182–10188
GaAzquez JL, MeAndez F (1994) J Phys Chem 98:4591–4593
Krishnamurti S, Roy RK, Vetrivel R, Iwata S, Pal S (1997) J Phys Chem A 101:7253–7257
Lee C, Yang W, Parr RG (1988) J Mol Struct (theochem) 163:305–313
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chermahini, A.N., Hosseinzadeh, B., Salimi Beni, A. et al. Theoretical studies on the reactivity of mono-substituted imidazole ligands. Struct Chem 25, 583–592 (2014). https://doi.org/10.1007/s11224-013-0322-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-013-0322-1