Abstract
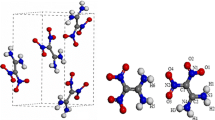
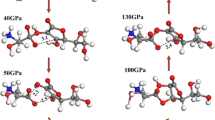
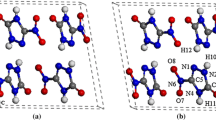
Periodic density functional theory calculations have been performed to study the structural, electronic, absorption, and thermodynamic properties of crystalline α-RDX under hydrostatic compression of 0–50 GPa. As the pressure increases, its lattice parameters, bond lengths, bonds angels, torsion angles, cell volumes, and band structure crystal change regularly except at the pressure of 13 GPa, where a structural transformation occurs. The remarkable changes in the bond lengths and bond angles indicate that there are several possible initiation decomposition mechanisms of RDX under compression. An analysis of density of states shows that the interactions between electrons, especially for the valence electrons, are strengthened under the influence of pressure. The absorption spectra show that the structural transformation makes the absorption coefficient of C–H stretching increase significantly. An analysis of thermodynamic properties indicates that the structural transformation is endothermic and not spontaneous at room temperature. The increasing temperature is not favorable for the structural transformation.










Similar content being viewed by others
References
Copper PW, Kurowski SR (1996) Introduction to the technology of explosives. Wiley, New York
Akhaven J (1998) The chemsitry of explosives. Royal Society of Chemistry, Cambridge
Dreger ZA, Gupta YM (2010) J Phys Chem A 114:8099–8105
Dreger ZA, Gupta YM (2007) J Phys Chem B 111:3893–3903
Miao MS, Dreger ZA, Winey JM, Gupta YM (2008) J Phys Chem A 112:12228–12234
Munday LB, Chung PW, Rice BM, Solares SD (2011) J Phys Chem B 115:4378–4386
Ciezak JF, Jenkins TA, Liu ZX, Hemley RJ (2007) J Phys Chem A 111:59–63
Swadley MJ, Li TL (2007) J Chem Theory Comput 3:505–513
Kuklja MM, Kunz AB (1999) J Phys Chem B 103:8427–8431
Chakraborty D, Muller RP, Dasgupta S, Goddard WA (2000) J Phys Chem A 104:2261–2272
Rice BM, Chabalowski CF (1997) J Phys Chem A 101:8720–8726
Harris NJ, Lammertsma K (1997) J Am Chem Soc 119:6583–6586
Wu CJ, Fried LE (1997) J Phys Chem A 101:8675–8679
Dreger ZA, Gupta YM (2012) J Phys Chem A 116:8713–8717
Ciezak JA, Trevino SF (2006) J Phys Chem A 110:5149–5155
Sorescu DC, Rice BM, Thompson DL (1999) J Phys Chem B 103:6783–6790
Patterson JE, Dreger ZA, Gupta YM (2007) J Phys Chem B 111:10897–10904
Baer BJ, Oxley J, Nicol M (1990) High Press Res 2:99–108
Byrd EFC, Rice BM (2007) J Phys Chem C 111:2787–2796
Zhu WH, Zhang XW, Wei T, Xiao HM (2009) Theor Chem Acc 124:179–186
Zhu WH, Zhang XW, Zhu W, Xiao HM (2008) Phys Chem Chem Phys 10:7318–7323
Zhu WH, Xiao JJ, Xiao HM (2006) Chem Phys Lett 422:117–121
Zhu WH, Xiao HM (2006) J Phys Chem B 110:18196–18203
Zhu WH, Xiao HM (2008) J Comput Chem 29:176–184
Zhu WH, Xiao HM (2010) Struct Chem 21:657–665
Segall MD, Lindan PJD, Probert MJ, Pickard CJ, Hasnip PJ, Clark SJ, Payne MC (2002) J Phys Condens Matter 14:2717–2744
Vanderbilt D (1990) Phys Rev B 41:7892–7895
Kresse G, Furthmüller J (1996) Phys Rev B 54:11169–11186
Fletcher R (1980) Practical methods of optimization vol 1. Wiley, New York
Ceperley DM, Alder BJ (1980) Phys Rev Lett 45:566–569
Perdew JP, Zunger A (1981) Phys Rev B 23:5048–5079
Choi CS (1972) Acta Cryst Sect B 28:2857–2862
Zhu WH, Xiao HM (2009) J Phys Chem B 113:10315–10321
Zhu WH, Wei T, Zhu W, Xiao HM (2008) J Phys Chem A 112:4688–4693
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865–6868
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Phys Rev B 46:6671–6687
Davidson AJ, Oswald IDH, Francis DH, Lennie AR, Marshall WG, Millar DIA, Pulham CR, Warren JE, Cumming AS (2008) Cryst Eng Commun 10:162–165
Zhao XS, Hintsa EJ, Lee YT (1988) J Chem Phys 88:801–810
Isayev O, Gorb L, Qasim M, Leszczynski J (2008) J Phys Chem B 112:10005–11013
Behrens R, Bulusu S (1992) J Chem Phys 96:8877–8891
Behrens R, Bulusu S (1992) J Chem Phys 96:8891–8897
Botcher TR, Wight CA (1994) J Chem Phys 98:5441–5444
Goto N, Fujihisa H, Yamawaki H, Eakabayashi K, Nakayama Y, Yoshida M, Koshi M (2006) J Phys Chem B 110:23655–23659
Liu H, Zhao J, Wei D, Gong Z (2006) J Chem Phys 124:124501–124510
Zhu WH, Xiao JJ, Ji GF, Zhang F, Xiao HM (2007) J Phys Chem B 111:12715–12722
Meyer R, Köhler J, Homburg A (2002) Explosive. Wiley-VCH, GmbH, Weiheim
Xu XJ, Zhu WH, Xiao HM (2007) J Phys Chem B 111:2090–2097
Kuklja MM, Stefanovich EV, Kunz AB (2000) J Chem Phys 112:3417–3423
Luty T, Ordon P, Eckhardt CJ (2000) J Chem Phys 117:1775–1784
Saha S, Sinha TP, Mookerjee A (2000) Phys Rev B 62:8828–8834
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 21273115 and U1230120) and the Grant from the National Key Laboratory of Shock Wave and Detonation Physics, the Institute of Fluid Physics, China Academy of Engineering Physics (Grant No. 076100-1197F).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Q., Zhu, W. & Xiao, H. Periodic DFT study of structural, electronic, absorption, and thermodynamic properties of crystalline α-RDX under hydrostatic compression. Struct Chem 25, 451–461 (2014). https://doi.org/10.1007/s11224-013-0306-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-013-0306-1