Abstract
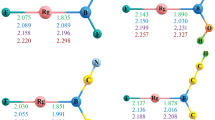
The geometries, atomic charge distributions, vibrational frequencies, and relative energies of the noble gas molecular anions XAuNgX− and HAuNgX− (X = F, Cl, Br; Ng = Xe, Kr, Ar) were investigated at the MP2 and CCSD(T) levels of theory. The Au–Ng bond length of X(H)AuNgX− is mainly dependent on the electronegative fragment bonded to the Au atom rather than on that bonded to the Ng atom. The presence of the right X− anion stabilizes the Au–Ng bond of X(H)AuNg. Based on the interatomic distances and atomic charge distributions, X(H)AuNgX− may be better described as X(H)AuNg···X− rather than as X(H)−···AuNgX. The MP2 calculations indicate that, for the Xe, Kr, and Ar molecular anion series, (i) X(H)AuNgX− is less stable than the global minimum X(H)AuX− + Ng by ca. 25–35, 33–48, and 37–57 kcal/mol, respectively, (ii) the reaction barriers are ca. 5–14, 3–9, and 2–5 kcal/mol, respectively, when the anion dissociates into X(H)AuX− + Ng through the bending transition state, and (iii) X(H)AuNgX− is more stable than the dissociation limit X(H)AuNg + X− by ca. 14–38, 11–30, and 9–25 kcal/mol, respectively.













Similar content being viewed by others
References
Bartlett N (1962) Xenon hexafluoroplatinate(V) Xe+[PtF6]−. Proc Chem Soc 218
Claassen HH, Selig H, Malm JG (1962) Xenon tetrafluorides. J Am Chem Soc 84:3593
Hoppe R, Dähne W, Mattauch H, Rödder K (1962) Fluorination of xenon. Angew Chem Int Ed 1:599
Frenking G, Cremer D (1990) The chemistry of the noble gas elements helium, neon, and argon-experimental facts and theoretical predictions. Struct Bonding 73:17–95
Christe KO (2001) A renaissance in noble gas chemistry. Angew Chem Int Ed 40:1419–1421
Grochala W (2007) Atypical compounds of gases, which have been called ‘noble’. Chem Soc Rev 36:1632–1655
Khriachtchev L, Räsänen M, Gerber RB (2009) Noble-gas hydrides: new chemistry at low temperatures. Acc Chem Res 42:183–191
Pyykkö P (1995) Predicted chemical bonds between rare gases and Au+. J Am Chem Soc 117:2067–2070
Schröder D, Schwarz H, Hrušák J, Pyykkö P (1998) Cationic gold(I) complexes of xenon and of ligands containing the donor atoms oxygen, nitrogen, phosphorus, and sulfur. Inorg Chem 37:624–632
Zeng T, Klobukowski M (2008) Relativistic model core potential study of the Au+Xe system. J Phys Chem A 112:5236–5242
Dixon DA, Wang TH, Grant DJ, Peterson KA, Christe KO, Schrobilgen GJ (2007) Heats of formation of krypton fluorides and stability predictions for KrF4 and KrF6 from high level electronic structure calculations. Inorg Chem 46:10016–10021
Grant DJ, Wang TH, Dixon DA, Christe KO (2010) Heats of formation of XeF3 +, XeF3 −, XeF5 +, XeF7 +, XeF7 −, and XeF8 from high level electronic structure calculations. Inorg Chem 49:261–270
Seidel S, Seppelt K (2000) Xenon as a complex ligand: the tetra xenono gold(II) cation in AuXe4 2+(Sb2F11 −)2. Science 290:117–118
Hu WP, Huang CH (2001) The intrinsic stability of the noble gas-coordinated transition-metal complex ions. J Am Chem Soc 123:2340–2343
Drews T, Seidel S, Seppelt K (2002) Gold–xenon complexes. Angew Chem Int Ed 41:454–456
Hwang IC, Seidel S, Seppelt K (2003) Gold(I) and mercury(II) xenon complexes. Angew Chem Int Ed 42:4392–4395
Gerken M, Moran MD, Mercier HPA, Pointner BE, Schrobilgen GJ, Hoge B, Christe KO, Boatz JA (2009) On the XeF+/H2O system: synthesis and characterization of the xenon(II) oxide fluoride cation, FXeOXeFXeF+. J Am Chem Soc 131:13474–13489
Evans CJ, Gerry MCL (2000) The microwave spectra and structures of Ar–AgX (X = F, Cl, Br). J Chem Phys 112:1321–1329
Evans CJ, Gerry MCL (2000) Noble gas–metal chemical bonding? The microwave spectra, structures, and hyperfine constants of Ar–CuX (X = F, Cl, Br). J Chem Phys 112:9363–9374
Evans CJ, Lesarri A, Gerry MCL (2000) Noble gas–metal chemical bonds. Microwave spectra, geometries, and nuclear quadrupole coupling constants of Ar–AuCl and Kr–AuCl. J Am Chem Soc 122:6100–6105
Evans CJ, Rubinoff DS, Gerry MCL (2000) Noble gas–metal chemical bonding: the microwave spectra, structures and hyperfine constants of Ar–AuF and Ar–AuBr. Phys Chem Chem Phys 2:3943–3948
Michaud JM, Cooke SA, Gerry MCL (2004) Rotational spectra, structures, hyperfine constants, and the nature of the bonding of KrCuF and KrCuCl. Inorg Chem 43:3871–3881
Thomas JM, Walker NR, Cooke SA, Gerry MCL (2004) Microwave spectra and structures of KrAuF, KrAgF, and KrAgBr; 83Kr nuclear quadrupole coupling and the nature of noble gas-noble metal halide bonding. J Am Chem Soc 126:1235–1246
Cooke SA, Gerry MCL (2004) XeAuF. J Am Chem Soc 126:17000–17008
Michaud JM, Gerry MCL (2006) XeCu covalent bonding in XeCuF and XeCuCl, characterized by Fourier transform microwave spectroscopy supported by quantum chemical calculations. J Am Chem Soc 128:7613–7621
Lovallo CC, Klobukowski M (2003) Transition metal–noble gas bonding: the next frontier. Chem Phys Lett 368:589–593
Belpassi L, Infante I, Tarantelli F, Visscher L (2008) The chemical bond between Au(I) and the noble gases. Comparative study of NgAuF and NgAu+ (Ng = Ar, Kr, Xe) by density functional and coupled cluster methods. J Am Chem Soc 130:1048–1060
Ghanty TK (2005) Insertion of noble-gas atom (Kr and Xe) into noble-metal molecules (AuF and AuOH): are they stable? J Chem Phys 123:074323
Ghanty TK (2006) How strong is the interaction between a noble gas atom and a noble metal atom in the insertion compounds MNgF (M = Cu and Ag, and Ng = Ar, Kr, and Xe)? J Chem Phys 124:124304
Li TH, Mou CH, Chen HR, Hu WP (2005) Theoretical prediction of noble gas containing anions FNgO− (Ng = He, Ar, and Kr). J Am Chem Soc 127:9241–9245
Borocci S, Bronzolino N, Grandinetti F (2008) Noble gas–sulfur anions: a theoretical investigation of FNgS− (Ng = He, Ar, Kr, Xe). Chem Phys Lett 458:48–53
Borocci S, Bronzolino N, Grandinetti F (2009) Noble gas–selenium molecular species: a theoretical investigation of FNgSe− (Ng = He–Xe). Chem Phys Lett 470:49–53
Antoniotti P, Borocci S, Bronzolino N, Cecchi P, Grandinetti F (2007) Noble gas anions: a theoretical investigation of FNgBN− (Ng = He − Xe). J Phys Chem A 111:10144–10151
Krouse IH, Hao CT, Check CE, Lobring KC (2007) Bonding and electronic structure of XeF3 −. J Am Chem Soc 129:846–852
Vasdev N, Moran MD, Tuononen HM, Chirakal R, Suontamo RJ, Bain AD, Schrobilgen GJ (2010) NMR spectroscopic evidence for the intermediacy of XeF3 − in XeF2/F− exchange, attempted syntheses and thermochemistry of XeF3 − salts, and theoretical studies of the XeF3 − anion. Inorg Chem 49:8997–9004
Sun YL, Hong JT, Hu WP (2010) Theoretical prediction of stable noble-gas anions XeNO2 − and XeNO3 − with very short xenon–nitrogen bond lengths. J Phys Chem A 114:9359–9367
Jayasekharan T, Ghanty TK (2008) Prediction of metastable metal-rare gas fluorides: FMRgF (M = Be and Mg; Rg = Ar, Kr and Xe). J Chem Phys 128:144314
Jiménez-Halla CÓC, Fernández I, Frenking G (2009) Is it possible to synthesize a neutral noble gas compound containing a Ng–Ng Bond? A theoretical study of H–Ng–Ng–F (Ng = Ar, Kr, Xe). Angew Chem Int Ed 48:366–369
Liu GQ, Yang Y, Zhang WX (2010) Theoretical study on the CH3NgF species. Struct Chem 21:197–202
Liu GQ, Li H, Zhang XX, Zhang WX (2011) Theoretical study on the RXeXeR′ species. Comput Theor Chem 963:394–402
Liu GQ, Zhou HQ, Wang L, Zhang XX, Zhang WX (2011) Molecular structure and infrared spectra of (HXeCN) n (n = 2, 3 or 4). Spectrochim Acta A 79:1105–1108
Liu GQ, Wang L, Zhang XX, Zhang WX (2011) Theoretical investigation of the XHgXeX and HHgXeX (X = F, Cl, Br) compounds. Comput Theor Chem 974:31–36
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.02. Gaussian Inc, Wallingford, CT
Møller C, Plesset MS (1934) Note on an approximation treatment for many-electron systems. Phys Rev 46:618–622
Frisch MJ, Head-Gordon M, Pople JA (1990) A direct MP2 gradient method. Chem Phys Lett 166:275–280
Frisch MJ, Head-Gordon M, Pople JA (1990) Semi-direct algorithms for the MP2 energy and gradient. Chem Phys Lett 166:281–289
Pople JA, Head-Gordon M, Raghavachari K (1987) Quadratic configuration interaction. A general technique for determining electron correlation energies. J Chem Phys 87:5968–5975
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80:3265–3269
Nicklass A, Dolg M, Stoll H, Preuss H (1995) Ab initio energy-adjusted pseudopotentials for the noble gases Ne through Xe: calculation of atomic dipole and quadrupole polarizabilities. J Chem Phys 102:8942–8952
Andrae D, Haeussermann U, Dolg M, Stoll H, Preuss H (1990) Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor Chem Acc 77:123–141
Martin JML, Sundermann A (2001) Correlation consistent valence basis sets for use with the Stuttgart–Dresden–Bonn relativistic effective core potentials: the atoms Ga–Kr and In–Xe. J Chem Phys 114:3408–3420
Mulliken RS (1955) Electronic population analysis on LCAO-MO molecular wave functions I. J Chem Phys 23:1833–1840
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor–acceptor viewpoint. Chem Rev 88:899–926
Cordero B, Gómez V, Platero-Prats AE, Revés M, Echeverría J, Cremades E, Barragán F, Alvarez S (2008) Covalent radii revisited. Dalton Trans 2832–2838
Pyykkö P, Atsumi M (2009) Molecular single-bond covalent radii for elements 1–118. Chem Eur J 15:186–197
Pyykkö P, Atsumi M (2009) Molecular double-bond covalent radii for elements Li-E112. Chem Eur J 15:12770–12779
Pyykkö P, Riedel S, Patzschke M (2005) Triple-bond covalent radii. Chem Eur J 11:3511–3520
Liu YL, Chang YH, Li TH, Chen HR, Hu WP (2007) Theoretical study on the noble-gas anions F−(NgO) n (Ng = He, Ar, and Kr). Chem Phys Lett 439:14–17
Lai TY, Yang CY, Lin HJ, Yang CY, Hu WP (2011) Benchmark of density functional theory methods on the prediction of bond energies and bond distances of noble-gas containing molecules. J Chem Phys 134:244110
Lignell A, Khriachtchev L, Lundell J, Tanskanen H, Räsänen M (2006) On theoretical predictions of noble-gas hydrides. J Chem Phys 125:184514
Winter M (2012) WebElements, http://www.webelements.com/sodium/atoms.html. Accessed 8 Jan 2012.
Winter M (2012) WebElements, http://www.webelements.com/fluorine/atoms.html. Accessed 8 Jan 2012.
Kapustinskii AF (1956) Lattice energy of ionic crystals. Q Rev Chem Soc 10:283–294
Winter M (2012) WebElements, http://www.webelements.com/sodium/atom_sizes.html. Accessed 8 Jan 2012.
Hicks WT (1963) Evaluation of vapor–pressure data for mercury, lithium, sodium, and potassium. J Chem Phys 38:1873–1880
Hildenbrand DL, Hall WF (1962) The vapor pressure and heat of sublimation of gold. J Phys Chem 66:754–755
DeCorpo JJ, Steiger RP, Franklin JL, Margrave JL (1970) Dissociation energy of F2. J Chem Phys 53:936–938
Acknowledgments
This study was supported by the Natural Science Research Foundation of the Education Department of Henan Province of China (Grant No. 2009A150032), by the Basic and Frontier Technical Research Project of Henan Province of China (Grant No. 102300410202), by the National Basic Research Program of China (Grant No. 2011CBA00701), and by the National Natural Science Foundation of China (Grant No. 21171084). Four anonymous reviewers are greatly acknowledged for helping us improving the original manuscript.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, G., Zhang, Y., Bai, X. et al. Theoretical investigation of the noble gas molecular anions XAuNgX− and HAuNgX− (X = F, Cl, Br; Ng = Xe, Kr, Ar). Struct Chem 23, 1693–1710 (2012). https://doi.org/10.1007/s11224-012-9978-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-012-9978-1