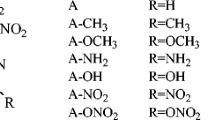Abstract
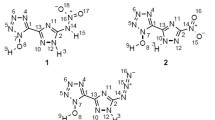
Density functional theory calculations at the B3LYP/aug-cc-pVDZ level have been performed to explore the structure, stability, heat of explosion, density, and the performance properties of amino-, methyl-, and nitroimidazoles. N-Nitroimidazoles have shown lower densities compared with those of C-nitroimidazoles. Detonation properties of title compounds were evaluated by using Kamlet–Jacob semi-empirical equations based on the predicted densities and the calculated heats of detonation. It has been found that some compounds with the calculated densities 2.0 g/cm3, detonation velocities over 9.10 km/s and detonation pressures of about 45 GPa (some even over 50 GPa) may be novel potential high energy materials. The higher performance of nitroimidazole-N-oxides is apparently due to their higher densities (2.0–2.515 g/cm3). Heat of explosion, stability, density, and performance properties are related to the number and relative positions of –NO2, –NH2, and –CH3 groups of the imidazole ring. The designed nitroimidazoles satisfy the criteria of high energy materials.


Similar content being viewed by others

References
Pagoria PF, Lee JS, Mitchell AR, Schmidt RD (2002) Thermochim Acta 384:187–204
Klapötke TM (2011) Chemistry of high-energy materials. Walter de Gruyter, Berlin
Gao H, Shreeve JM (2011) Chem Rev 111:6513–6556
Dave PR, Duddu R, Yang K, Damavarpu R, Gelber N, Surapaneni R, Gilardi R (2004) Tetrahedron Lett 45:2159–2162
Duddu R, Dave PR, Damavarapu R, Surapaneni R, Gilardi R (2005) Synth Commun 35:2709–2714
Damavarapu R, Surapaneni R, Duddu R, Farohor F, Dave PR, Parrish D (2007) J Heterocycl Chem 44:241
Duddu R, Dave PR, Damavarapu R, Surapaneni R, Gilardi R, Parrish D (2008) Synth Commun 38:767–774
Duddu R, Dave PR, Damavarapu R, Surapaneni R, Parrish D (2009) Synth Commun 39:4282–4288
Duddu R, Dave PR, Damavarapu R, Gelber N, Parrish D (2010) Tetrahedron Lett 51:339–401
Raja D, Zhang M-X, Damavarapu R, Gelberb N (2011) Synthesis 17:2859–2864
Cho JR, Kim KJ, Cho SG, Kim JK (2002) J Heterocycl Chem 3:141–144
Katritzky AR, Cundy DJ, Chen J (1993) J Energ Mater 11:345–352
Gao H, Ye C, Gupta OD, Xiao JC, Hiskey MA, Twamley B, Shreeve JM (2007) Chem Eur J 13:3853–3860
Grimmett MR, Hua S, Chang R, Foley S, Simpson J (1989) Aust J Chem 42:1281–1289
Bracuti AJ (1995) J Chem Crystallogr 25:625–627
Pinkerton AA, Zhuorva EA, Chen Y-S (2003) In: Politzer P, Murray JS (eds) Energetic materials, theoretical and computational chemistry series. Elsevier, New York
Larina L, Lopyrev V (2009) Nitroazoles. Synthesis, structure and application. Springer, Berlin
Ravi P, Badgujar DM, Gore GM, Tewari SP, Sikder AK (2011) Prop Explos Pyrotech 36:393–403
Ravi P, Gore GM, Tewari SP, Sikder AK (2012) J Mol Model 18:597–605
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, Revision B.04. Gaussian, Inc., Pittsburgh, PA
Turker L, Avalar T, Gumus S, Namur Y (2009) J Hazard Mater 167:440–448
Turker L, Avalar T (2009) J Hazard Mater 162:193–203
Ravi P, Gore GM, Tewari SP, Sikder AK (2010) J Hazard Mater 183:859–865
Njegic B, Gordon MS (2006) J Chem Phys 125:224102–224112
Materials Studio 4.1 (2004) Accelrys Inc., San Diego, CA
Kamlet MJ, Jacobs SJ (1968) J Chem Phys 48:23–25
Akhavan J (1998) Chemistry of explosives. The Royal Society of Chemistry, Cambridge
Zhang C, Shu Y, Huang Y, Zhao X, Dong H (2005) J Phys Chem B 109:8978–8982
Zhang C (2009) J Hazard Mater 161:21–28
Zhang C, Shu Y, Wang X, Zhao X, Tan B, Peng R (2005) J Phys Chem A 109:6592–6596
Zhi C, Cheng X (2010) Prop Explos Pyretech 35:555–560
Fukui F, Yonezawa T, Shingu H (1952) J Chem Phys 20:722–725
Zhou Z, Parr RG (1990) J Am Chem Soc 112:5720–5724
Pearson RG (1989) J Org Chem 54:1423–1440
Goh EM, Cho SG, Park BS (2000) J Def Tech Res 6:91–96
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbe A (2009) Mol Phys 107:2095–2101
Kim CK, Cho SG, Kim CK, Park H-Y, Zhang H, Lee HW (2008) J Comput Chem 29:1818–1824
Belsky VK, Zorkii PM (1977) Acta Crystallogr Sect A 13:1004–1006
Craven BM, Mcmullen RK, Bell JD, Freeman HC (1977) Acta Crystallogr Sect B 33:2585–2589
Eaton PE, Gilardi R, Zhang M-X (2000) Adv Mater 12:1143–1148
Hoffman DM (2003) Prop Explos Pyrotech 28:194–200
Mader CL (1998) Numerical modeling of explosives and propellants, 2nd edn. CRC Press, Boca Raton, FL
Politzer P, Murray JS (2011) Central Eur J Energ Mater 8(3):209–220
Depluech A, Cherville J (1978) Prop Explos Pyrotech 3:169–175
Depluech A, Cherville J (1979) Prop Explos Pyrotech 4:121–129
Xiao HM (1994) Molecular orbital theory of nitro compounds. Publishing House of Defense Industry, Peking
Kamlet MJ, Adolph HG (1979) Prop Explos Pyrotech 4:30–34
Mullay J (1987) Prop Explos Pyrotech 12:60–63
Politzer P, Murray JS (1995) Mol Phys 86:251–255
Murray JS, Concha MC, Politzer P (2009) Mol Phys 107:89–97
Rice BM, Hare JJ (2002) J Phys Chem A 106:1770–1780
Brinck T, Murray JS, Politzer P (1992) Mol Phys 76:609–617
Murray JS, Lane P, Politzer P (1995) Mol Phys 85:1–8
Murray JS, Lane P, Politzer P (1998) Mol Phys 93:187–194
Pospìŝil M, Vávra P, Concha MC, Murray JS, Politzer P (2010) J Mol Model 16:895–901
Zeman S (1999) J Energ Mater 17:305–330
Zeman S (2006) J Hazard Mater 132:155–164
Skimmer D, Olson D, Block-Bolton A (1997) Prop Explos Pyrotech 23:34–42
Wang G, Xiao H, Ju X, Gong X (2006) Prop Explos Pyrotech 31:102–109
Wang G, Xiao H, Ju X, Gong X (2006) Prop Explos Pyrotech 31:361–368
Hess BA, Schaad LJ (1971) J Am Chem Soc 93:2413–2416
Zhou Z, Parr RG, Garst JF (1988) Tetrahedron Lett 29:4843–4846
Zhou Z, Parr RG (1989) J Am Chem Soc 111:7371–7379
Acknowledgments
We are grateful to the referees for enlightening comments and useful suggestions. We thank Defense Research Development Organization, India for the financial assistance through Advanced Centre of Research in High Energy Materials.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravi, P., Tewari, S.P. Theoretical investigations on the structure, density, thermodynamic and performance properties of amino-, methyl-, and nitroimidazoles and their N-oxides. Struct Chem 23, 1953–1970 (2012). https://doi.org/10.1007/s11224-012-0028-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-012-0028-9