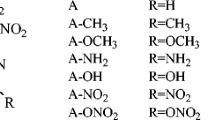Abstract
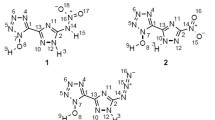
We explore herein the structure, stability, heat of explosion, density, and the performance properties of amino, nitro, and nitroso substituted tetrazoles and their N-oxides using the density functional theory calculations at the B3LYP/aug-cc-pVDZ level. N-Nitro compounds have lower densities compared with those of C-nitrotetrazoles. Kamlet-Jacob semi-empirical equations were used to calculate the performance properties of designed compounds. The higher performance of tetrazole-N-oxides is due to their higher densities (2.110–2.287 g/cm3). Heat of explosion, stability, density and performance properties are related to the number and relative positions of NO2, NH2, and NO groups of the tetrazole ring. The designed molecules satisfy the criteria of high energy materials.

Similar content being viewed by others
References
Klapötke TM (2007) In: Klapötke TM (ed) High energy density materials. Springer, Berlin
Klapötke TM, Miro-Sabate C, Welch JM (2008) Z Anorg Allg Chem 634:857
Klapötke TM, Mayer P, Miro-Sabae C, Welch JM, Wiegand N (2008) Inorg Chem 47:6014
von Denffer M, Klapötke TM, Miro-Sabate C (2008) Z Anorg Allg Chem 634:2575
Klapötke TM, Miro-Sabate C, Welch JM (2008) Dalton Trans p 6372
Stierstorfer J, Klapötke TM, Hammerl A, Chapman B (2008) Z Anorg Allg Chem 634:1051
Klapötke TM, Stierstorfer J (2007) Helv Chim Acta 90:2132
Klapötke TM, Miro-Sabate C (2007) Z Anorg Allg Chem 633:2671
Darwich C, Klapötke TM, Miro-Sabate C (2008) Chem Eur J 14:5756
Ye C, Xiao J C, Twamley B, Shreeve J M (2005) Chem Commun p 2750
Xue H, Arritt SW, Twamley B, Shreeve JM (2004) Inorg Chem 43:7972
Xue H, Gao Y, Twamley B, Shreeve JM (2005) Chem Mater 17:191
Xue H, Gao Y, Twamley B, Shreeve JM (2005) Inorg Chem 44:5068
Gao Y, Twamley B, Shreeve JM (2006) Chem Eur J 12:9010
Zhang C, Wang X, Huang H (2008) J Am Chem Soc 130:8359
Molchanova MS, Pivina TS, Arnautova EA, Zefirov NS (1999) J Mol Struct 465:11
Jia-Rong L, Jian-Min Z, Hai-Shan D (2005) J Chem Crystallogr 35:943
Churakov AM, Tartakovsky VA (2004) Chem Rev 104:2601
Inagake S, Goto N (1987) J Am Chem Soc 109:3234
Noyman M, Zilberg S, Haas Y (2009) J Phys Chem A 113:7376
Liepa AJ, Jones DA, Mc Carthy TD, Nearn RH (2000) Aust J Chem 53:619
Harel T, Rozen S (2010) J Org Chem 75:3141
Göbel M, Karaghisosoff K, Klapötke TM, Piercy DG, Stierstorfer J (2010) J Am Chem Soc 132:17216
Fukui F, Yonezawa T, Shingu H (1952) J Chem Phys 20:722
Materials Studio Version 4.1 (2004) Accelrys Inc., San Diego
Kamlet MJ, Jacobs SJ (1968) J Chem Phys 48:23
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery AJ, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain M, Farkas CO, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, revision B.04. Gaussian Inc., Pittsburgh
Ravi P, Gore GM, Venkatesan V, Tewari SP, Sikder AK (2010) J Hazard Mater 183:859
Ravi P, Shee SK, Gore GM, Tewari SP, Sikder AK (2011) Struct Chem 22:661
Ravi P, Gore G M, Tewari S P, Sikder A K (2011) J Mol Model. doi:10.1007/s00894-011-1099-z
Njegic B, Gordan MS (2006) J Chem Phys 125:224102
Akhavan J (1998) In: Chemistry of explosives, The Royal Society of Chemistry, Cambridge
Larina L, Lopyrev V (2009) In: Nitroazoles: synthesis, structure and application, Springer, New York
Zhou Z, Parr RG (1990) J Am Chem Soc 112:5720
Pearson RG (1989) J Org Chem 54:1423
Pinkerton AA, Zhuorva EA, Chen Y-S (2003) In: Politzer P, Murray JS (eds) Energetic materials, theoretical and computational chemistry series. Elsevier, New York
Goh EM, Cho SG, Kim JK (2001) In: A novel QSPR method to estimate densities of energetic materials, 222nd ACS National Meeting, Chicago, 26–30 Aug 2001
Ammon HL (2001) Struct Chem 21:205
Sorescu DC, Rice BM, Thompson DL (2000) J Phys Chem B 104:8406
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbe A (2009) Mol Phys 107:2095
Kim CK, Cho SG, Kim CK, Park HY, Zhang H, Lee H (2008) J Comput Chem 29:1818
Belsky VK, Zorkii PM (1977) Acta Crystallogr Sect A 13:1004
Lide DR (2006) CRC handbook of chemistry and physics, 87th edn. CRC Press, Boca Raton
Hoffman DM (2003) Prop Explos Pyrotech 28:194
Eaton PE, Gilardi R, Zhang MX (2000) Adv Mater 12:1143
Politzer P, Murray JS (2011) Cen Eur Energ Mater 8:209
Acknowledgments
We are very grateful to the referees for their useful suggestions. The first author acknowledges the sustaining financial support from Defense Research Development Organization (DRDO), India through Advanced Centre of Research in High Energy Materials (ACRHEM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravi, P., Tewari, S.P. A DFT study on the structure–property relationship of amino-, nitro- and nitrosotetrazoles, and their N-oxides: new high energy density molecules. Struct Chem 23, 487–498 (2012). https://doi.org/10.1007/s11224-011-9898-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-011-9898-5