Abstract
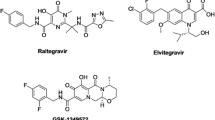
It is known that the HIV-1 integrase (IN) strand transfer inhibitors include the chelating fragments forming the coordinating bonds with two Mg2+ ions placed in the IN active site. The subject of the article is the role of these coordination bonds on stability of ligand–IN complexes. For this purpose, a set of ligand–IN complexes was investigated theoretically and experimentally. The theoretical model is based on the quantum-chemistry calculations of coordinating bonds geometry and energy. Solvent effects were taking into account using the implicit water model and the two-stage calculation scheme developed previously. For the experimental part of our study a set of the ligands was synthesized, and their IC50 values of IN inhibiting have been measured. It is shown that the main contribution to ligand–IN complexes stability is caused by the substitution of water molecules by the ligand in the first coordination sphere of two Mg2+ ions, and the change in the polarization energy of the bulk water. It is shown, that acid–base equilibrium and tautomeric forms of the ligands should be taken into account to improve the prediction ability of the theoretical estimations. All these factors are controlled by the chelating fragments of the ligands. It is demonstrated that our theoretical approach based on the consideration of the coordinating bonds allows to separate active ligands (inhibitors) from inactive ones.



Similar content being viewed by others
References
Pace P, Di Francesco ME, Gardelli C, Harper S, Muraglia E, Nizi E, Orvieto F, Petrocchi A, Poma M, Rowley M, Scarpelli R, Laufer R, Gonzalez Paz O, Monteagudo E, Bonelli F, Hazuda D, Stillmock KA, Summa V (2007) J Med Chem 50:2225–2239
Pommier Y, Johnson AA, Marchand C (2005) Nat Rev Drug Discov 4:236–248
Hare S, Gupta SS, Valkov E, Engelman A, Cherepanov P (2010) Nature 464:232–236
Jiao D, King C, Grossfield A, Darden TA, Ren P (2006) J Phys Chem B 110:18553–18559
Ponder JW, Wu C, Ren P, Pande VS, Chodera JD, Schnieders MJ, Haque I, Mobley DL, Lambrecht DS, DiStasio RA, Head-Gordon JM, Clark GNI, Johnson ME, Head-Gordon T (2010) J Phys Chem B 114:2549–2564
Gresh N, Cisneros GA, Darden TA, Piquemal J-P (2007) J Chem Theory Comput 3:1960–1986
Nunthaboot N, Pianwanit S, Parasuk V, Ebalunode JO, Briggs JM, Kokpol S (2007) Biophys J 93:3613–3626
Alves CN, Marti S, Castillo R, Andres J, Moliner V, Tunon I, Silla EA (2007) Chem Eur J 13:7715–7724
Puerta DT, Lewis JA, Cohen SM (2004) J Am Chem Soc 126:8388–8389
Puerta DT, Cohen SM (2003) Inorg Chem 42:3423–3430
Vanommeslaeghe K, Loverix S, Geerlingsb P, Tourwe D (2005) Bioorgan Med Chem 13:6070–6082
Tomasi J, Persico M (1994) Chem Rev 94:2027–2094
Liao C, Nicklaus MC (2010) ChemMedChem 5:1053–1066
Grigor’ev FV, Golovacheva AYu, Romanov AN, Kondakova OA, Sulimov VB (2009) Russ J Phys Chem A 83:565–574
Nikitina E, Sulimov V, Grigoriev F, Kondakova O, Luschekina S (2006) Int J Quantum Chem 106:1943–1963
Remko M (1997) Mol Phys 91:929–936
Vallet V, Wahlgren U, Grenthe I (2003) J Am Chem Soc 125:14941–14950
Henderson LJ (1908) Am J Physiol 21:173–179
Hasselbalch KA (1917) Biochem Z 78:112–144
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Cossi M, Rega N, Scalmani G, Barone V (2003) J Comput Chem 24:669–681
Leh H, Brodin P, Bischerour J, Deprez E, Tauc P, Brochon JC, LeCam E, Coulaud D, Auclair C, Mouscadet JF (2000) Biochemistry 39:9285–9294
Rice PA, Baker TA (2001) Nat Struct Biol 8:302–307
Chiu TK, Davies DR (2006) Front Med Chem 3:3–22
Davies DR, Braam LM, Reznikoff WS, Rayment I (1999) J Biol Chem 274:11904–11913
Davies DR, Goryshin IY, Reznikoff WS, Rayment I (2000) Science 289:77–85
Steiniger-White M, Rayment I, Reznikoff WS (2004) Curr Opin Struct Biol 14:50–57
Ason B, Knauss DJ, Balke AM, Merkel G, Skalka AM, Reznikoff WS (2005) Antimicrob Agents Chemother 49:2035–2043
Barecca ML, DeLuca L, Iraci N, Chimirri A (2006) J Med Chem 49:3994–3997
Allen FH (2002) Acta Crystallogr Sect B 58:380–388
Acknowledgments
This work was supported by the Russian Foundation for Basic Research (grants no. 08-04-12129-ofi, 09-04-93108CNRS) and Victory Pharmaceutical Ltd, Moscow, Russia.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A
Let’s consider the Henderson–Hasselbalch equation [17, 18] for the equilibrium of the charged (A−) and neutral (AH) states of the molecule:
The ΔG exp is defined as:
where [A] = [AH] + [A−], [AP] = [AHP] + [A−P] (P is the protein). Because only ionized product form the chelate complex with protein, [AHP] ≪ [A−P]. So the can rewrite (7) as
On the other hand,
From (6):
so
Appendix B
The free energy of the formation of the IN–ligand complexes ΔG b is expressed in terms of the molar concentration [I], [L], [LI] of the IN, ligand, and complexes IN–ligand, respectively:
where \( K_{\text{d}} = \frac{[{\text LI}]}{[{\text I}][{\text L}]} \) is the equilibrium constant.
Rewrite (12) as
where [I]0 is the total concentration of the IN which is fixed under the experiments with different inhibitors, [L(IC50)] is the total concentration of the inhibitor providing the double decreasing of the IN activity.
It can be shown that the [LI] remains constant for different inhibitors if the total concentration of the substrate [S]0 is fixed in the experiments for the [L(IC50)] determination. Let us consider the total free energy F of the system containing N I, N S, N L, N SI, N LI molecules of the IN, substrate, ligand in unbound state and complexes IN–substrate and IN–ligand, respectively:
where the total partition function Z in the dilute solution limit can be expressed as:
where Z I is the individual partition function of the IN, other terms in (15) are defined on the similar way, Z 0 is the part of the Z not dependent on N I, N S, N L, N SI, N LI. Since
only two independent variables are in (14): N LI and N SI. The equilibrium condition is:
Using (15–18) and Stirling’s approximation \( \ln \,N! \cong N(\ln \,N - 1) \) for large N, we obtain:
and
where V, N A is the total volume of the system and Avogadro number, respectively. Using (16) rewrite (19) as
On the similar way, we can obtain for the system without ligands with same numbers of the molecules of the IN and substrate:
Since, the left parts of (20) and (21) are equal, we obtain:
Since the [L(IC50)] provides the double decreasing of IN activity, \( [{\text{SI}}] = \frac{{[{\text{SI}}]_{\text{pure}} }}{2} \) and the [LI] in (22) depends only on [S]0, [I]0, [SI].
So using (13) we can write for two different ligands L1, L2:
In (23), we take into account that values of [LI], [I]0 do not depend on ligand. Under the condition [L2(IC50)], [L1(IC50)] ≫ [LI] (23) can be rewritten as:
The expression (24) is used in this work for the comparison of the experimental and calculated relative stability of the IN–ligand complexes.
Rights and permissions
About this article
Cite this article
Grigoriev, F.V., Golovacheva, A.Y., Romanov, A.N. et al. Stability of HIV-1 integrase–ligand complexes: the role of coordinating bonds. Struct Chem 23, 185–195 (2012). https://doi.org/10.1007/s11224-011-9855-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-011-9855-3