Abstract
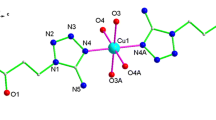
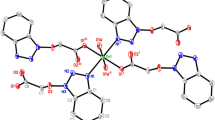
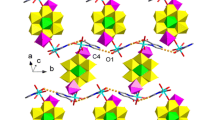
Three copper(II), one zinc(II), and one ferrous(II) complexes having 3-bromo or 3,8-dibromo-1,10-phenanthroline ligand with different metal/ligand molar ratios, formulated as [Cu(3-bromo-phen)(ClO4)(C3H7NO)2(H2O)](ClO4) (1), [Cu(3,8-dibromo-phen)(ClO4)(C3H7NO)2(H2O)](ClO4) (2), [Cu(3,8-dibromo-phen)(ClO4)(H2O)3](ClO4)(H2O)3 (3), [Zn(3,8-dibromo-phen)2(H2O)2](ClO4)2(H2O)2 (4), and [Fe(3,8-dibromo-phen)3](ClO4)2(H2O)(CH4O)(C3H6O)2 (5) (phen = 1,10-phenanthroline), have been synthesized and characterized in this paper. X-ray single-crystal diffraction studies reveal the different crystallographic symmetry and packing fashions between neighboring phen rings in 1:1 Cu(II) complexes 1–3 due to the alteration of bromo substituent 1,10-phenanthroline ligands and coordinated or free solvent molecules. Additionally, in 1:2 Zn(II) and 1:3 Fe(II) complexes 4 and 5, continuous π–π stacking and alternating π–π and dimeric p–π stacking are found.
Graphical abstract
Five mononuclear complexes having 3-bromo or 3,8-dibromo-1,10-phenanthroline ligand with different molar ratios of metal and ligand have been spectrally and structurally characterized, where different one-dimensional π–π stacking modes are found in the crystal packing of 1, 3, 4, and 5.







Similar content being viewed by others
References
Tsutsui T, Nakada N, Saito S, Ogino E (1994) Appl Phys Lett 65:1868
Saitoh Y, Yamamoto T (1995) Chem Lett 785
Okada K, Uekawa M, Wang YF, Chen TW, Nakaya T (1998) Chem Lett 801
Higari MA, Birckner E, Heise B, Klemm EJ (1999) Polym Sci A Polym Chem 37:4442
Ng K, Gong X, Chan SH, Lam LSM, Chan WK (2001) Chem Eur J 7:4358
Yasuda T, Yamaguchi I, Yamamoto T (2003) Adv Mater 15:293
Yamamoto T, Saitoh Y, Anzai K (2003) Macromolecules 36:6722
Yasuda T, Yamamoto T (2003) Macromolecules 36:7513
Yamamoto T, Anzai K, Iijima T (2004) Macromolecules 37:3064
Suzuki H, Kanbara T, Yamamoto T (2004) Inorg Chim Acta 357:4335
Hartwig JF (1998) Angew Chem Int Ed 37:2046
Muci AR, Buchwald SL (2002) Top Curr Chem 219:131
Tzalis D, Tor Y (1995) Tetrahedron Lett 36:6017
Toshi HS, Jamshidi R, Tor Y (1999) Angew Chem Int Ed 38:2722
Miller MT, Karpishin TB (1999) Inorg Chem 38:5246
Araki K, Endo H, Masuda G, Ogawa T (2004) Chem Eur J 10:3331
Huang W, Tanaka H, Ogawa TJ (2008) Phys Chem C 112:11513
Wang L, You W, Huang W, Wang C, You XZ (2009) Inorg Chem 48:4295
Huang W, Masuda G, Tanaka H, Ogawa T (2008) Inorg Chem 47:468
Huang, W.; Wang, L.; Tanaka, H.; Ogawa, T. Eur. J. Inorg. Chem. 2009, 1321
Wang L, You W, Huang W (2009) J Mol Struct 920:270
Bush PM, Whitehead JP, Pink CC, Gramm EC, Pence LE (2001) Inorg Chem 40:1871
Titze C, Kaim W, Zalis S (1997) Inorg Chem 36:2505
Durand J, Zangrando E, Stener M, Fronzoni G, Milani B (2006) Chem Eur J 12:7639
Aligo JA, Smith L, Eglin JL, Pence LE (2005) Inorg Chem 44:4001
Chen CY, Lu HC, Wu CG, Chen JG, Ho KC (2007) Adv Funct Mater 17:29
Rau S, Fischer R, Jäger M, Schäfer B, Meyer S, Kreisel G, Görls H, Rudolf M, Henry W, Vos JG (2004) Eur J Inorg Chem 2001
Lee KJ, Yoon I, Lee SS, Lee BY (2002) Bull Korean Chem Soc 23:399
Saitoh Y, Koizumi T, Osakada K, Yamamoto T (1997) Can J Chem 75:1336
Molecular Structure Corporation & Rigaku Corporation (2001) Crystalclear Version 1.3. MSC, 9009 New Trails Drive, The Woodlands, TX 77381-5209, USA and Rigaku, Toyko, Japan
Molecular Structure Corporation & Rigaku Corporation (2000) TEXSAN. Version 1.11. MSC, 9009 New Trails Drive, The Woodlands, TX 77381-5209, USA and Rigaku, Toyko, Japan
Bruker SHELXTL (Version 6.10) (2000) Bruker AXS Inc., Madison, Wisconsin, USA
Wang ZX, Huang BY, Wang SM, Xue RJ, Huang XJ, Chen LQ (1997) Electrochim Acta 42:261l
Flack HD (1983) Acta Crystallogr A 39:876
Acknowledgments
W.H. acknowledges the Major State Basic Research Development Programs (Nos. 2007CB925101, 2011CB933300, and 2011CB808704), the National Natural Science Foundation of China (No. 20871065) and the Jiangsu Province Department of Science and Technology (No. BK2009226).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, F., Hu, B., Tao, T. et al. Supramolecular frameworks composed of copper(II), zinc(II), and ferrous(II) complexes having 3-bromo or 3,8-dibromo-1,10-phenanthroline ligand with different molar ratios of metal and ligand. Struct Chem 22, 123–133 (2011). https://doi.org/10.1007/s11224-010-9702-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-010-9702-y