Abstract
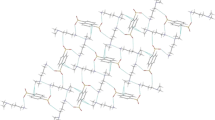
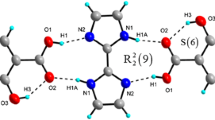
The molecular and crystal structure of a 1:1 co-crystal of 4,4′-dimethyl-7,7′-bi([1,2,5]thiadiazolo[3,4-b]pyridylidene)–chloranilic acid, (1), has been determined by X-ray diffraction at the monoclinic space group P21/c with cell parameters of a = 8.422(6), b = 7.343(4), c = 16.112(7) Å, β = 104.988(8)°, V = 962.5(10) Å3 and Z = 2. In the crystal structure, two components connect via the intermolecular O–H···N hydrogen bonds [2.804(4) Å] and S···O heteroatom interaction [2.945(3) Å] with R 2 2(7) couplings to form a unique and infinite one-dimensional supramolecular tape structure. The calculations of (1) at the HF/6-31G(d), MP2/6-31G(d), and B3LYP/6-31G(d) levels can almost reproduce X-ray geometry. In addition, the distances of the intermolecular O–H···N and S···O interactions by MP2/6-31G(d) and B3LYP/6-31G(d) levels agree well with those in the crystal. The calculated binding energies corrected BSSE and ZPE are −4.487 (HF), −7.473 (MP2), and −5.640 (B3LYP) kcal/mol. The results suggest that the complex (1) is very stable and the dispersion interaction is significantly important for the attractive intermolecular interaction in (1). The NBO analysis has revealed that the n(N) → σ*(O–H) interaction gives the strongest stabilization to the system and the major interaction for the intermolecular S···O contact is n(O) → σ*(S–N).
Index Abstract
In the crystal structure of the title compound, the molecules are linked by intermolecular O–H···N hydrogen bonds and short S···O heteroatom interactions with R 2 2(7) couplings to construct a unique and infinite one-dimensional supramolecular tape structure.





Similar content being viewed by others
References
Desiraju GR, Steiner T (1999) The weak hydrogen bond in structural chemistry and biology. Oxford University Press, Oxford
Hibbert F, Emsley J (1990) Adv Phys Org Chem 26:255. doi:10.1016/S0065-3160(08)60047-7
Desiraju GR (1995) Angew Chem Int Ed Engl 34:2311. doi:10.1002/anie.199523111
Braga D, Grepioni F (eds) (2007) Making crystals by design. Wiley-VCH, Weinheim
Williams JM, Ferraro JR, Thorn RJ, Carlson KD, Geiser U, Wang HH, Kini AM, Whangbo M-H (1992) Organic superconductors (including fullerenes). Prentice Hall, New Jersey
Nalwa HS (ed) (1997) Handbook of organic conductive molecules and polymers, vol 1. Wiley, Chichester
Yamashita Y, Tomura M (1998) J Mater Chem 8:1933. doi:10.1039/a803151g
Yamashita Y, Ono K, Tomura M, Tanaka S (1997) Tetrahedron 53:10169. doi:10.1016/S0040-4020(97)00356-6
Yamashita Y, Ono K, Tomura M, Imaeda K (1997) Chem Commun (Camb) 1851. doi:10.1039/a704375i
Zaman MB, Tomura M, Yamashita Y (2000) Org Lett 2:273. doi:10.1021/ol991229q
Zaman MB, Tomura M, Yamashita Y (2001) J Org Chem 66:5987. doi:10.1021/jo001746i
Tomura M, Yamashita Y (2008) Anal Sci 24:x31. doi:10.2116/analscix.24.x31
Hadzi D (ed) (1997) Theoretical treatment of hydrogen bonding. Wiley, Chichester
Scheiner S (1997) Hydrogen bonding: a theoretical perspective. Oxford University Press, Oxford
Buckingham AD, Fowler PW, Hutson JM (1988) Chem Rev 88:963. doi:10.1021/cr00088a008
Jeziorski B, Moszynski R, Szalewicz K (1994) Chem Rev 94:1887. doi:10.1021/cr00031a008
Rovira C, Novoa JJ (1999) Chem Eur J 5:3689. doi:10.1002/(SICI)1521-3765(19991203)5:12<3689::AID-CHEM3689>3.0.CO;2-H
Iwaoka M, Takemoto S, Okada M, Tomoda S (2002) Bull Chem Soc Jpn 75:1611. doi:10.1246/bcsj.75.1611
Burling FT, Goldstein BM (1993) Acta Crystallogr B 49:738. doi:10.1107/S0108768193000709
Sheldrick GM (2008) Acta Crystallogr A 64:112. doi:10.1107/S0108767307043930
Spek AL (2003) J Appl Cryst 36:7. doi:10.1107/S0021889802022112
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, van J (2006) J Appl Cryst 39:453. doi:10.1107/S002188980600731X
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision E.01. Gaussian Inc., Wallingford, Connecticut
Møller C, Plesset MS (1934) Phys Rev 46:618. doi:10.1103/PhysRev.46.618
Head-Gordon M, Pople JA, Frisch MJ (1988) J Chem Phys Lett 153:503. doi:10.1016/0009-2614(88)85250-3
Becke D (1993) J Chem Phys 98:5648. doi:10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785. doi:10.1103/PhysRevB.37.785
Jian FF, Zhao PS, Wang QX (2005) Chin J Struct Chem 24:184
Lyakhov AS, Matulis VE, Gaponik PN, Voitekhovich SV, Ivashkevich OA (2008) J Mol Struct 876:260. doi:10.1016/j.molstruc.2007.06.027
Boys SF, Bernardi F (1970) Mol Phys 19:53. doi:10.1080/00268977000101561
Reed AE, Weinhold F (1983) J Chem Phys 78:4066. doi:10.1063/1.445134
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1993) NBO Version 3.1. Theoretical Chemistry Institute, University of Wisconsin, Madison, WI
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor R (1987) J Chem Soc Perkin Trans 2:S1. doi:10.1039/p298700000s1
Mellini M, Merlino S (1976) Acta Crystallogr B 32:1074. doi:10.1107/S056774087600469X
Bernstein J, Davis RE, Shimoni L, Chang N-L (1995) Angew Chem Int Ed Engl 34:1555. doi:10.1002/anie.199515551
Weber E (ed) (1998) Design of organic solids. Springer-Verlag, New York
Allen FH (2002) Acta Crystallogr B 58:380. doi:10.1107/S0108768102003890
Laurent G, Durant F (1981) Crystallogr Struct Commun 10:1015
Verenich AI, Govorova AA, Galitskii NM, Potkin VI, Kaberdin RV, Ol’dekop YA (1992) Khim Get Soedin SSSR 399
Wierzejewska M, Saldyka M (2004) Chem Phys Lett 391:143. doi:10.1016/j.cplett.2004.04.101
Neuheuser T, Hess BA, Reutel C, Weber E (1994) J Phys Chem 98:6459. doi:10.1021/j100077a007
Jaffe RL, Smith GD (1996) J Chem Phys 105:2780. doi:10.1063/1.472140
Meijer EJ, Sprik M (1996) J Chem Phys 105:8684. doi:10.1063/1.472649
Tsuzuki S, Lüthi HP (2001) J Chem Phys 114:3949. doi:10.1063/1.1344891
Taylor R, Kennard O (1982) J Am Chem Soc 104:5063. doi:10.1021/ja00383a012
Acknowledgments
The authors thank the Instrument Center, Institute for Molecular Science, Okazaki, Japan, for the X-ray crystallographic analysis and Research Center for Computational Science, Okazaki Research Facilities, National Institutes of Natural Sciences, Okazaki, Japan, for the computations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomura, M., Ono, K. & Yamashita, Y. Crystallographic and theoretical studies of 4,4′-dimethyl-7,7′-bi([1,2,5]thiadiazolo[3,4-b]pyridylidene)–chloranilic acid (1/1) with intermolecular O–H···N hydrogen bonds and S···O heteroatom interactions. Struct Chem 19, 967–974 (2008). https://doi.org/10.1007/s11224-008-9382-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-008-9382-z