Abstract
The multiple analytes produced during the operation of nuclear facilities are required to monitor the smooth operation of the plant in the environment of high temperature and radioactivity in real time. A chemiresisitive electronic nose was investigated and developed to analyze the multiple analytes generated in the nuclear reactor/allied facilities. An electronic nose consists of chemiresisitive sensor, array, housing, hardware, software, and pattern recognition algorithm. The sensor and array of different semiconductor metal oxides were prepared, processed, and developed to sense the multiple analytes. The hardware and data acquisition software (DAS) was designed and developed to acquire the dynamic responses from the array of four sensors. The hardware provides a low excitation voltage for measurement of the dynamic response of four sensors towards the improvement of the life of the sensor. The various experiments were conducted with multiple analytes at different temperatures to study the analysis of analytes. The performance of the hardware and DAS were tested and evaluated with the sensor array responses towards three analytes, viz., hydrogen (H2), formaldehyde (HCHO), and hydrazine (NH2NH2). Different features evaluated from the response traces were processed to teach the instrument using pattern recognition algorithms. The training and real-time testing of the sensor array realized the qualitative discrimination and quantitative estimation of the analytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Detecting multiple analytes is very important for the smooth operation of plants and taking preventive action towards improving the efficiency of plants [1]. Many industrial processes, viz., production of drugs, agriculture, medical, military, chemicals, food products, beverages, etc., involve using several reactants as the input for production [2,3,4,5]. In these industries, the quality of the product is evaluated by its purity and the presence of by-products in it. The progress of the process is also monitored at various critical points in these industries by an offline or online collection of samples as required [6,7,8]. When the species involved in the evaluation are gaseous in nature, either mass spectrometry or gas chromatography is employed, which measures the species offline and is bulky [9, 10]. The methods specified as examples provide a qualitative and quantitative account of the species evaluated [11, 12]. On most occasions, combinations of techniques or methodologies are resorted in providing accurate and reliable values, enabling the operators to continue the process in an uninterrupted fashion [13, 14]. An alternate technique that provides both a qualitative and quantitative analysis of analytes in real-time is the electronic nose, which is based on the human nose methodology [15,16,17,18,19,20]. Designing an electronic nose using a sensor array with differentially selective sensors has the advantage of using responses from all the sensors over a single sensor that adds new dimensions to the observation, which may help to estimate multiple analytes more accurately [21,22,23,24,25,26,27]. Data from the sensor array help distinguish different analytes and measure their concentration using pattern recognition techniques [28,29,30,31,32]. The change in electrical resistance/conductance of semiconducting metal oxides (SMOs) upon exposure to different gases is employed for chemical sensing [33,34,35]. The variations in operating temperature, nature of dopant, and processing methodologies will significantly vary the selectivity toward different analytes due to varying sensing characteristics [36,37,38]. Significant contributions toward the development of an electronic nose based on SMOs are available in various literatures for particular applications in laboratory level [39,40,41]. Depending on the application concerned, the hardware/software of the e-nose alters and calls for investigation, study, design, development, testing/validation, training, and deployment [42, 43]. Such an approach requires the unique design and development of interface electronic modules for data acquisition systems, data banks, data processing, and comparison algorithms for qualitative and quantitative estimation of the species. There are various methods that measure the responses from e-nose that, include signal conditioning and data processing using a multiplexer (MUX), an analog-to-digital converter (ADC), and an 8051 processor and pattern recognition technique for qualitative and quantitative analysis of analytes [44,45,46,47]. Application-oriented e-noses for perfume odour classification, onion quality evolution, low-cost indoor air quality monitoring, determination of environmental odours, detection E-coli in drinking water, etc., were reported [48,49,50,51,52,53,54].
In the nuclear industry, different gaseous by-products are generated during the operation of different nuclear/allied facilities like spent fuel reprocessing plants, waste management plants, heavy water production plants, etc. [55, 56]. A currently off-line analytical technique is being employed for their detection and is required for the investigation of detection and quantification of multiple analytes in real time.
This paper describes an investigation, design, and development of an electronic nose setup for the discrimination and quantification of analytes of interest for nuclear allied areas in an environment of high temperature, and radioactivity. A chemiresisitive sensor array of four SMOs were processed, and developed and corresponding responses vary dynamically with the change of concentration of analytes. The sensor array was loaded in an in-house designed chamber. Hardware was designed and developed to measure the dynamic sensor array responses using signal conditioning and data processing units by exciting all the sensors with low voltages to improve the life of the sensor and sends data to PC through local area network (LAN). Data acquisition software was developed for logging, monitoring, and online/offline visualization of the four channels of data through LAN and had various graphical features to visualize and study the behavior of sensors towards analytes. The developed hardware and software were tested and validated with multiple analytes by operating the sensor array at different temperatures. Different characteristics from sensor array response were evaluated and analyzed by principal component analysis (PCA) and principal component regression (PCR) algorithm that were developed in the software. Different properties of sensor response were studied, and investigated the best feature for the training of the instrument for the qualitative and quantitative analysis of multiple analytes of hydrogen (A1), formaldehyde (A2), and hydrazine (A3). The instrument was taught from the experimental data using pattern recognition algorithms and was able to discriminate and quantify the multiple analytes in t real time.
2 Overall Scheme of Electronic Nose
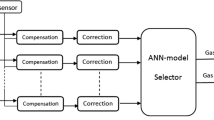
The scheme (Fig. 1) for an electronic nose consists of a sensor array assembly with a programmable power supply, signal conditioning and data processing unit, ethernet interface, data acquisition, and pattern recognition algorithms for the qualitative and quantification of analytes.
The sensor array consists of four sensors from S1 to S4 with different SMOs. The multichannel hardware measures the dynamic response from four channels. The qualitative and quantitative analyses of multiple analytes were executed by applying a pattern recognition algorithm on sensor array data.
3 Sensor Array and Housing
3.1 Sensing Materials Deployed in Current Study
The SMO gas sensor works on the principle of chemoresistance. Four different SMO sensors, viz., indium oxide (S1), tin oxide (S2), zinc oxide (S3), and chromium niobate (S4), were prepared and processed. The sensors of different materials were designed and developed for the sensing of A1, A2, and A3 analytes. Among them, CrNbO4 is a p-type SMO, while others are n-type. The resistance of n-type material decreases in the presence of a reducing analyte while, it increases for a p-type material.
3.2 Design and Development of Semiconductor Metal Oxide Sensors
A screen printing technique was employed to develop the sensor for the current investigation. In this technique, the sensing material, and heater were prepared, processed and printed on the sensor base in the specific pattern using print screen mesh followed by heat treatment [57,58,59,60,61,62]. The details of in-house development and fabrication of semiconductor metal oxide sensors of different materials using screen printing are described in Sect. 1 of supplementary material.
3.3 Design and Development of Sensor Array Housing
The sensors’ housing was designed and developed to accommodate four sensors. The details of mechanical drawing (SF2.1) are discussed in Sect. 2 of the supplementary material. The thick films of S1, S2, S3 and S4 were loaded in the housing. The typical sensor array housing for the four sensors of array is presented in Fig. 2.
All four sensors' heaters were connected to the multi-channel programmable DC power supply and maintained at optimized temperatures. The gaseous analytes were injected through a leak-tight septum, and the instrument developed in-house measured corresponding sensor array responses based on the constant voltage method. The different features (sensitivity, differential signal rise, and recovery times) were derived from the responses and considered for analysis.
3.4 Methodology for Assessing the Performance of the Sensor
Different concentrations of analytes A1, A2, and A3 ranging from 10 to 500 ppm were considered for the current study. The analytes were injected by a Hamilton microliter gas tight syringe through a septum. After attaining a steady response due to analytes injection, the housing valve was left open for natural air diffusion to replenish fresh ambiance in the chamber without any forced carrier flow. The sensor response changed from the baseline when the analyte was injected and retraced back to the baseline when the chamber outlet was open to air.
4 Design and Development of Hardware for Measurement of Sensor Array Responses
The hardware was designed and developed to measure the dynamic responses from the four sensors. The in-house developed hardware provides a low excitation voltage of 100 to 300 mV across the sensor, which improves the sensor life. This hardware also offers high accuracy, stability, input impedance, reliability, etc. Typically, the range of sensor response is 10 to 10000 kΩ towards analyte concentrations from 10 to 500 ppm. As the response of the sensor changes with the concentration of the analytes, the hardware was designed for broad dynamic characteristics of sensors towards analytes and low excitation voltage across the sensors so that the life of the sensor will not degrade. The developed hardware excites all the sensors of the array and measures the responses of the sensor using 8051 microcontroller-based signal conditioning and data processing unit. The signal conditioning unit processes all the sensors response and converts them into engineering units using algebraic equations by calibration. The hardware sends the array data through LAN, where data can be acquired and stored in a file for post-analysis of sensors towards analytes. The hardware was simulated and optimized using multisim software.
The circuit diagram of 4-channel hardware based on constant voltage and corresponding photograph is presented in Fig. 3.
5 Software Scheme
5.1 Data Acquisition Software for the Sensor Array of Four Sensors
Data acquisition software is used to store the sensor data in a file for post-analysis [63,64,65]. In the current study, the protocol of the in-house developed hardware is different and is required to process and acquire the sensor array data of four sensors into the PC remotely in a file through LAN. Typically, the user can program the number of average data points, delay time between the samplings, file name to save the data, etc. The quad-channel data acquisition software is presented in Fig. 4. The software configures and initiates the LAN communication with the hardware, and the acquired data was saved in a user-chosen file. The data received was processed and converted into the response of the sensor. The software was developed to communicate with the hardware using suitable commands, processes using suitable string conversion functions, and error handling for an error-free and generous program flow. The error handling was executed into the main program in such a way that any error would not lead to a crash or hanging of the program. The program displays the sensors’ online time-series data. The program was tested and validated with standard inputs. The acquired sensor array data saved in the file can be directly imported into the text file for further processing. The software also provides the off-line graph as the popup window, where the user can select the desired channel to view a large amount of data.
5.2 Development of Pattern Recognition Algorithm
Earlier, authors implemented PCA to recognize analytes from responses from a 3-sensor array using an astable multivibrator based instrument [66]. In the current study, the pattern recognition algorithm was extended to process the data from a 4-sensor array along with the development of PCR for quantitative analysis. The details of the development of PCA and PCR algorithms in the software for the sensor array of four sensors are provided in Sect. 3 of the supplementary material.
6 Gas Sensing Studies with 4-Sensor Array Towards Multiple Analytes
Typically, semiconducting metal oxides chemisorb oxygen at high temperature that reacts with the analyte gases and alters the charge carrier density, manifesting as the electrical resistance changes.
The various experiments were conducted with different concentrations of analytes A1, A2, and A3 at different temperatures, and measured corresponding dynamic responses. The sensor response was measured as a function of the temperature in range of 548 to 598 K with an interval of 25 K. The optimum temperature required for the operation of sensor towards the highest sensitivity was found to be 325 ºC. A typical variation of sensitivity with temperature for the sensor was presented in Fig. 5. All sensors were operated at the same temperature to minimize the process variable-dependent relation among the responses.
The sensor array with sensors S1 to S4 was evaluated in the air at an operating temperature of 325 °C. Initially, the baseline stability was recorded continuously, and a typical recording for about eight hours is presented in Fig. 6, with corresponding means and standard deviations shown in the inset. The testing of the sensor array with analytes A1 to A3 in the concentration range from 10 to 500 ppm individually was followed by measuring the corresponding responses using developed instrumentation. All the analyte injections were repeated thrice for concurrence, and the average value was considered for analysis.
The rise time is described as the time interval over which response of the sensor towards gas reaches a fixed percentage (usually 90%) of the final value when the sensor is exposed to the gas at a full scale concentration. Recovery time is the time interval over which the sensor response towards gas reduces to 10% of the saturation value when the sensor is exposed to full scale concentration of the gas and then placed in clean air ambient.
The typical responses of S3, S1, and S4 toward different concentrations of A1, A2, and A3, respectively, are shown in Fig. 7(a–c).
The variations in different characteristics, viz., sensitivity, differential signal, rise time, and recovery time derived from the responses of the sensors are detailed in Figs. SF4.1, SF4.2, and SF4.3 of Sect. 4 of the supplementary material.
The developed sensor array will be used in chemical handling of reprocessing facilities of nuclear reactor, where the gas sampling interval will be about 30 min to qualify the air ambience. The recovery time of 5–7 min will not pose any problem of interference between the successive samples for the intended application in real-time monitoring.
7 Application of Pattern Recognition Algorithms
7.1 Qualitative Discrimination of Analytes
Among different characteristics derived from the sensor array responses, the sensitivity of four sensors from S1 to S4 towards multiple analytes (A1 to A3) with different concentrations is presented in Fig. 8.
In the current study, the features matrices consisting of 33 observations (analytes with varying concentrations) with four variables (sensors) from the experiments were analyzed using the PCA module described in Sect. 5. The PCA was carried out on different properties of sensor response for the qualitative analysis of analytes, and the corresponding all Eigen values and principal components were computed and saved in the database for training the instrument.
The selectivity of the sensors towards different analytes summarizing the output from Fig. 8 was presented in Table 1. The details of sensitivity vs. gas concentration are described in the supplementary material of Sect. 4.
A typical scree plot to screen the number of principal components (PCs) for sensitivity data (Fig. SF5.1, Sect. 5 of supplementary materials) indicates that the 1st and 2nd PCs sufficiently show the maximum variation in data, and the corresponding score plot is shown in Fig. 9.
Similar analysis with other feature data matrices, viz., differential signal, rise time, and recovery time, resulted in corresponding patterns in 2D-PC space (Figs. SF5.5, SF5.6, and SF5.7 in Sect. 5 of the supplementary material). The percentages of corresponding PCs were tabulated accordingly in Table ST1 of supplementary material. Among the feature matrices considered, the sensitivity matrix could discriminate all three analytes without overlapping with 95.1% (1st PC 87.6%, 2nd PC 7.5%) of variation that discriminates A1, A2, and A3 in real time (Fig. 9).
7.2 Quantitative Analysis of Analytes
7.2.1 Data Training
From the sensitivity data matrix constructed for PCA, 70% of samples were considered for training the instrument, and the remaining 30% was taken for the validation of the PCR model.
The PCA scores output of training data was fetched as input for PCR, which gives more than 95% discrimination of analytes. The regression coefficient matrix (β) is related to the concentration matrix (C) through Eq. (1) and was computed. The estimated β values were stored in the database. The typical β values for different analytes measured from an in-house developed instrument are presented in Table 2.
For the quantification of analytes, the concentration of unknown can be computed using Eq. (2).
7.2.2 Validation of Electronic Nose
The performance of the electronic nose setup was evaluated with the remaining 30% sample data that corresponds to the 6 × 2 data matrix. A good linear correlation between the measured and the standard concentrations of hydrogen (Fig. 10) supports the validation of the PCR model in quantifying the analytes with 4-sensor array setup.
The linear fit details of all analytes A1, A2, and A3 between standard and measured concentrations are presented in Table 3. The developed setup is able to quantify the analytes with the accuracies mentioned in Table 2.
For the study of the sensor's life, the individual sensor’s materials performances were studied in detail for more than three months each. In the current study, the new sensor films of In2O3, SnO2, ZnO and CrNbO4 compositions were developed and studied for qualitative (PCA) and quantification analysis (PCR) of multiple analytes towards the nuclear reactor/allied facilities.
8 Conclusions
An electronic nose setup was investigated, designed and developed for the qualitative and quantitative analysis of A1 (H2), A2 (HCHO) and A3 (NH2NH2) using a sensor array of four sensors S1 (In2O3), S2 (SnO2), S3 (ZnO) and S4 (CrNbO4) and pattern recognition algorithm for the nuclear reactor/allied facilities. The sensors of different materials were prepared, processed, and developed to sense the multiple analytes generated in nuclear reactors. The instrumentation was developed in such way that it provides a low excitation voltage across the sensors to improve life of the sensor. The various experiments were carried out at different temperatures with different concentrations of multiple analytes. The PCA (principal component analysis) and PCR (principal component regression) of the pattern recognition algorithm was developed in the software. The algorithms were executed on different sensing features of sensor response (viz., sensitivity, differential signal, rise, and recovery time) measured from the developed instrument and investigated that sensitivity was the best among for the discrimination of analytes under study. The electronic nose was trained from the experimental data and evaluated for the quantitative analysis of these analytes using PCR. The measured and estimated values found a good correlation with a minimum regression coefficient of determination R2 = 0.997. The instrumentation setup was trained from the experimental data using a pattern recognition algorithm and is able to discriminate and quantify the multiple analytes of hydrogen (H2), formaldehyde (HCHO), and hydrazine (NH2NH2) with a maximum variance of 95.1% and an average accuracy of 98.1% respectively. This low-voltage excitation instrumentation would help extend the lifetime of the sensor, which would be undertaken as a part of the long-term performance of the sensor array on field applications. The customized instrument setup can be modified/tuned for other chemiresisitive- based sensor arrays for agriculture, medical, food, military, pharma industry applications, etc.
Data Availability
The data supporting this study's findings are available from the corresponding author upon reasonable request.
References
Muthuvinayagam, M., Meganathan, S., Jankiraman, S., & Dineshraja, S. (2014). Industrial gas monitoring with safety closure. International Journal of Engineering & Technology, 3, 1035–1040.
Baldwin, E. A., Bai, J., Plotto, A., & Dea, S. (2011). Electronic noses and tongues: Applications for the food and pharmaceutical industries. Sensors, 11, 4744–4766.
Zohora, S., Khan, A., Srivastava, A., Dey, N. (2016). Gas sensing techniques in electronic nose and its applications: A review. In 3rd International conference on electrical, electronics, engineering trends, communication, optimization and sciences (EEECOS) (pp. 178–183).
Loutfi, A., Coradeschi, S., Mani, G. K., Shankar, P., & Rayappan, J. B. B. (2015). Electronic noses for food quality: A review. Journal of Food Engineering, 144, 103–111.
Liu, L., Li, X., Li, Z., & Shi, Y. (2018). Application of electronic nose in detection of fresh vegetables freezing time considering odor identification technology. Chemical Engineering Transactions, 68, 265–270.
Wilson, A. D., & Baietto, M. (2009). Applications and advances in electronic-nose technologies. Sensors, 9, 5099–5148.
Bhandare, P. B., Pendbhaje, N. S., & Narang, A. P. (2013). Electronic nose: A review, research and reviews. Journal of Engineering and Technology, 2, 2324–2347.
Rock, F., Barsan, N., & Weimar, U. (2008). Electronic nose: Current and future trends. Chemical Reviews, 108, 705–725.
Wisniewska, P., Sliwinska, M., Dymerski, T., Wardencki, W., & Namiesnik, J. (2017). Comparison of an electronic nose based on ultrafast gas chromatography, comprehensive two-dimensional gas chromatography, and sensory evaluation for an analysis of type of whisky. Journal of Chemistry, 2017, 1–13.
Berna, A. Z., Trowell, S., Cynkar, W., & Cozzolino, D. (2008). Comparison of metal oxide-based electronic nose and mass spectrometry-based electronic nose for the prediction of red wine spoilage. Journal of agricultural and food chemistry, 01, 4–17.
Gao, D., Ji, J., & Cai, C. (2012). Quantitative analysis of different volatile organic compounds using an improved electronic nose. Measurement Science and Technology, 23, 1–10.
Hosseini, S. A., Akbarzadeh, M. R. T., & Sistani, M. B. (2016). Qualitative and quantitative evaluation of EEG signals in epileptic seizure recognition. International Journal of Intelligent Systems and Applications, 06, 41–46.
Hdrczyczak, E. G., Guzek, D., Moleda, Z., Kalinowska, I. W., Brodowska, M., & Wierzbicka, A. (2016). Applications of electronic noses in meat analysis. Food Science and Technology, 36, 389–395.
Montuschi, P., Mores, N., Trove, A., Mondino, C., & Barnes, P. J. (2013). The electronic nose in respiratory medicine. Respiration, 85, 72–84.
Gongora, A., Monroy, J., & Jimenez, J. G. (2018). An electronic architecture for multipurpose artificial noses. Journal of Sensors, 2018, 1–9.
Tan, J., & Xu, J. (2020). Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artificial Intelligence in Agriculture, 4, 104–115.
Karakaya, D., Ulucan, O., & Turkan, M. (2019). Electronic nose and its applications: A survey. International Journal of Automation and Computing, 17, 179–209.
Horczyczak, E. G., Guzek, D., Moleda, Z., Kalinowska, I. W., Brodowska, M., & Wierzbicka, A. (2016). Applications of electronic nose in meat analysis. Food Science and Technology, 36, 389–395.
Furizal, F., Maarif, A., Firdaus, A. A., & Rahmaniar, W. (2023). Future potential of E-nose technology: A review. International Journal of Robotics and Control Systems, 3, 449–469.
Jia, P., Li, X., Xu, M., & Zhang, L. (2024). Classification techniques of electronic nose: A review. International journal of Bio-Inspired computation, 23, 16–27.
Singh, S., Sajana, S., Varma, P., Sreelekha, G., Adak, C., Shukla, R. P., & Kamble, V. B. (2023). Metal oxide-based gas sensor array for VOCs determination in complex mixtures using machine learning. Microchimica Acta., 1, 19–96.
Su, P. G., & Li, M. C. (2021). Recognition of binary mixture of NO2 and No gases using a chemiresistive sensors array combined with principal componnet analysis. Sensors and Actuators A: Physical, 331, 112980.
Jimenez, J. G., Monroy, J. G., & Blanco, J. L. (2011). The Multi-chamber electronic nose-An improved olfaction sensor for mobile robotics. Sensors, 11, 6145–6164.
Kudarihal, C. S., & Gupta, M. (2014). Electronic nose based on metal oxide semiconductor sensors as an alternative technique for perception of odours. International Journal of Advances in Engineering & Technology, 7, 206–216.
Faleh, R., Bedoui, S., & Kachouri, A. (2020). Review on smart electronic nose coupled with artificial intelligence for air quality monitoring. Advances in Science Technology and Engineering Systems Journal, 5, 739–747.
Ozmen, A., & Dogan, E. (2009). Design of a portable E-nose instrument for gas classifications. IEEE Transactions on Instrumentation and Merasurement, 58, 3609–3618.
Tomchenko, A. A., Harmer, G. P., Marquis, B. T., & Allen, J. W. (2003). Semiconducting metal oxide sensor array for the selective detection of combustion gases. Sensors and Actuators B: Chemical, 93, 126–134.
Karyakarte, S., & Savant, I. (2019). Pattern recognition process, methods and applications in artificial intelligence. International Research Journal of Engineering and Technology, 6, 1162–1166.
Powell, M. A., & Thakor, N. V. (2013). A training strategy for learning pattern recognition control for myoelectric prostheses. Journal of Prosthetics and Orthotics: JPO, 25, 30–41.
Liu, A., Guo, L., Wang, M., Su, C., Wang, D., Dong, H., Chen, J., & Wu, W. (2023). Review on algorithm design in electronic noses: Challenges, status, and trends. Intelligent Computing, 12, 1–19.
Giorgi, M. G. D., Donateo, T., Ficarella, A., Menga, N., Chiodo, L. S., & Strafella, L. (2024). Coupling principal component analysis-based sensor data reduction techniques and multi-net systems for simultaneous prediction of multi-component degradation levels in hybrid electric rotorcraft engines. Measurement, 227, 1–24.
Chang, I. S., Byun, S. W., Lim, T. B., & Park, G. M. (2024). A study on E-nose system in terms of the learning efficiency and accuracy of boosting approaches. Sensors, 24, 1–12.
Becker, T., Ahlers, S., Braunmuhl, C. B., Muller, G., & Kiesewetter, O. (2001). Gas sensing properties of thin- and thick-film tin oxide materials. Sensors and Actuators B: Chemical, 77, 551–561.
Sun, Y. F., Liu, S. B., Meng, F. L., Liu, J. Y., Jin, Z., & Kong, L. T. (2012). Metal oxide nanostructures and their gas sensing properties: A review. Sensors (Basel, Switzerland), 12, 2610–2631.
Liu, X., Cheng, S., Liu, H., Hu, S., Zhang, D., & Ning, H. (2012). A survey on gas sensing technology. Sensors, 12, 9635–9665.
Rock, F., Barsan, N., Weimar, U. (2009). Metal oxide gas sensor arrays: geometrical design and selectivity. AIP Conference Proceeding p. 1137.
Marikutsa, A., Rumyantseva, M., Konstantinova, E. A., & Gaskov, A. (2021). The key role of active sites in the development of selective metal oxide materials. Sensors, 21, 1–44.
Wawrzyniak, J. (2023). Advancements in improving selectivity of metal oxide semiconductor gas sensors opening new perspectives for their application in food industry. Sensors, 23, 1–35.
Yi, T. H., Li, H. N., & Gu, M. (2011). A new method for optimal selection of sensor location on a high-rise building using simplified finite element model. Structural Engineering & Mechanics, 37, 1–10.
Tang, K. T., Pan, S. W. C. H., Hsieh, H. Y., Liang, Y. S., & Liu, S. C. (2010). Development of a portable electronic nose system for the detection and classification of fruity odors. Sensors, 10, 9179–9193.
Falasconi, M., Concina, I., Gobbi, E., Sberveglieri, V., Pulvirenti, A., & Sberveglieri, G. (2012). Electronic nose for microbiological quality control of food products. International Journal of Electrochemistry, 2012, 1–12.
Devi, D. A., Savithri, T. S., & Sugun, L. S. (2020). Design and implementation of real time data acquisition system using reconfigurable SoC. International Journal of Advanced Computer Science and Applications, 11, 325–331.
Xu, B. Q., Liu, T. X., Cheng, Q., Tang, M. G., Zheng, G., & Lei, H. (2024). Development of data acquisition software for electromagnetic instruments in landslide detection. Applied geophysics, 22, 133–146.
Berna, A. (2010). Metal oxide sensors for electronic noses and their application to food analysis. Sensors, 10, 3882–3910.
Borowik, P., Adamowicz, L., Tarakowski, R., Waclawik, P., Oszako, T., Slusarski, S., & Tkaczyk, M. (2021). Development of a low-cost electronic nose for detection of pathogenic fugi and applying it to fusarium oxysporum and rhizoctonia solani. Sensors, 21, 1–18.
Wang, L., Tan, Y., Cui, X., & Cui, H. (2012). The application of LabVIEW in data acquisition system of solar absorption refrigerator. Energy Procedia, 16, 1496–1502.
Wendi, X., Ling, Y., & Enliang, W. (2019). Application research of ethernet communication system based on UIP protocol stack. I. OP Conf. Series: Journal of Physics: Conf Series, 1237, 1–7.
Harun, F. K. C., Ibrahim, N. A., & Basri, M. A. M. (2011). Development of portable electronic nose device for perfume odour classification. Jurnal Teknologi, 54, 23–30.
Konduru, T., Rains, G. C., & Li, C. (2015). A customized metal oxide semiconductor-based gas sensor array for onion quality evaluation: System development and characterization. Sensors, 15, 1252–1273.
Zampolli, S., Elmi, I., Ahmed, F., Passini, M., Cardinali, G. C., Nicoletti, S., & Dori, L. (2004). An electronic nose based on solid state sensor arrays for low-cost indoor air quality monitoring applications. Sensors and Actuators B: Chemical, 101, 39–46.
Nayak, K., Supreetha, B. S., Deccaraman, M., & Nayak, V. (2012). E-Nose system to detect E-Coli in drinking water of Udupi District. International Journal of Engineering Research and Development, 1, 58–64.
Macasaet, D., Bandala, A., Illahi, A. A., Dadios, E., Lauguico, S., & Alejandrino, J. (2021). Development of electronic nose for smell categorization using artificial neural network. Journal of Advances in Information Technology, 12, 36–44.
Raj, M. P., Swaminarayan, P. R., Saini, J. R., & Parmar, D. K. (2015). Applications of pattern recognition algorithms in agriculture: A Review. International Journal of Advanced Networking and Applications, 6, 2495–2502.
Saii, M. M. (2019). Classification of pattern recognition techniques used deep learning and machine learning. International Journal of Computer Science Trends and Technology, 7, 165–173.
Zhen, Y. Q. (2016). Safety and effective developing nuclear power to realize green and low-carbon development. Advances in Climate Change Reaserch, 7, 10–16.
Wu, Z., Wang, H., Wang, X., Zheng, H., Chen, Z., & Meng, C. (2020). Development of electronic nose for qualitative and quantitative monitoring of volatile flammable liqids. Sensors, 20, 1–12.
Degueldre, C., Dawson, R., Cooley, I., & Besley, E. (2021). Fission gas released from molten salt reactor fuel: The case of nobel gas short life radioisotopes for radiopharmaceutical application. Medicine in Novel Technology and Devices, 10, 1–8.
Horvath, A., & Rachlew, E. (2016). Nuclear power in the 21st century: Challenges and possibilities. Ambio, 45, S38–S49.
Sree Rama Murthy, A., Gnanasekar, K. I., Jayaraman, V., Umarji, A. M., & Gnanasekaran, T. (2015). Conductometric sensing of H2 by chromium niobate (CrNbO4). IEEE Sensors Journal, 15, 7054–7060.
Prabhu, E., Jayaraman, V., Gnanasekar, K. I., Gnanasekaran, T., & Periaswami, G. (2005). Pulsed laser deposition made thin film sensor for monitoring hydrogen in gas streams. Asian Journal of Physics, 14, 33–40.
Shukla, T. (2012). Synthesis of tin oxide thick film and its investigation as a LPG sensor at room temperature. Journal of Sensor Technology, 2, 102–108.
Arshak, K., & Gaidan, I. (2005). Development of a novel gas sensor based on oxide thick films. Materials Science and Engineering B, 118, 44–49.
Keshari, A. K., Prabhakar Rao, J., & Sree Rama MurthyJayaraman, A. V. (2020). Design and development of instrumentation for the measurement of sensor array responses. Review of Scientific Instruments, 91, 1–9.
Zailan, Z., Kamat, R., Arfauz, M., & Rahman, A. (2016). A real-time data acquisition approach of environmental ergonomic parameter using LabVIEW. Journal of Engineering Science and Technology, 1, 40–47.
Yu, H., Meng, H., & Li, L. (2018). A multi-type data acquisition platform based on LabVIEW. IOP Conf. Series: Materials Science and Engineering, 466, 1–6.
Keshari, A. K., Prabhakar Rao, J., Sree Rama Murthy, A., & Jayaraman, V. (2019). Development of instrumentation setup for discrimination of analytes using principal component analysis. Engineering Research Express, 1, 1–11.
Funding
Open access funding provided by Department of Atomic Energy. The authors received no financial support for the research work.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to research work as follows: AKK, JPR, ASRM, and VJ. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keshari, A.K., Prabhakar Rao, J., Sree Rama Murthy, A. et al. Investigation of Chemiresisitive Electronic Nose for the Analysis of Multiple Analytes Using Pattern Recognition Algorithm. Sens Imaging 25, 37 (2024). https://doi.org/10.1007/s11220-024-00487-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11220-024-00487-0