Abstract
In 1988, Robert O’Hara coined the now ubiquitous phrase “tree thinking” to highlight the importance of cladistics for proper evolutionary reasoning. This accessible phrase has been taken up widely in the professional, popular, and educational literatures, and it has played an important role in helping spread phylogenetic thinking far beyond the disciplinary borders of systematics. However, the undeniable benefits of the spread of tree thinking have become marred by being widely linked to several misconceptions that were present in O’Hara’s original writings. O’Hara incorrectly considered clades to be the central subjects of evolutionary narratives. By failing to appreciate that clades contain independently evolving lineages, O’Hara has promoted the misleading view that evolution is irreducibly branched. In this paper, I show how an exclusive focus on the branching realm of taxa has created a cladistic blindfold that has caused a form of lineage blindness that has spread widely through the literature dedicated to the teaching of tree thinking. Its symptoms include the rejection of phenomena and concepts that are fundamental to the realm of evolving lineages, including linear evolutionary imagery and narratives, the concepts of anagenetic evolution and missing links, our evolutionary descent from monkeys and apes, and the promotion of the nonsensical concept of collateral ancestors. To avoid simplistic tree thinking, it is crucial to recognize that the realms of taxa and lineages have distinctive features that require different kinds of thinking. I close by suggesting that teaching can be improved by linking tree thinking explicitly to lineage thinking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Thirty-six years ago, Robert O’Hara (1988) coined the phrase “tree thinking” as a shorthand for proper thinking about evolutionary trees (O’Hara, 1988, 1992, 1998). Tree thinking emphasizes the branching nature of phylogeny, which provides the necessary context for correctly posing and answering questions about the origin and evolution of taxa and their traits. O’Hara’s ideas about tree thinking were inspired by the cladistic approach to systematics, which focuses on reconstructing the branching relationships between taxa. According to cladistic principles, all taxa are represented by terminal branches in tree-like diagrams, called cladograms. The diverging branches are connected by nodes that are interpreted as points of common ancestry (hypothetical common ancestors). Cladograms are concise visual summaries of the hierarchical patterns of relationships between taxa, but they do not depict direct ancestor–descendant relationships between taxa. However, by mapping the taxonomic distribution of organismal traits onto cladograms, researchers can make evolutionary inferences, including the order in which traits evolved. Interpreted in this way, cladograms function as evolutionary or phylogenetic trees that represent the diverging histories of evolving lineages.
O’Hara’s phrase and its associated ideas have become ubiquitous in the literatures aimed at professional, popular, and educational audiences, and they have greatly assisted in spreading cladistic ideas beyond the disciplinary borders of systematics. However, the salutary influence of the spread of tree thinking has been accompanied by the largely unrecognized spread of several misconceptions, the seeds of which O’Hara unwittingly sowed in his own writings. By promoting a virtually exclusive focus on the collateral relationships of terminal taxa that overlooks the linear realm of lineages, O’Hara inadvertently created what I like to call a cladistic blindfold. This has caused him and others to overlook the lineal nature of evolutionary descent and to promote evolutionary views that are theoretically untenable and contradicted by empirical evidence (Jenner, 2018, 2022). What is worrying is that these misleading views are especially common precisely where they can do the most harm, namely in sources aimed at lay and educational audiences.
There is a sizeable recent literature dedicated to teaching students and non-specialist audiences tree thinking, with much of it published in journals aimed at educators (Ainsworth & Saffer, 2013; Baum & Offner, 2008; Baum & Smith, 2013; Baum et al., 2005; Blacquiere et al., 2020; Brown, 2016; Catley & Novick, 2008; Catley et al., 2010, 2013; Danos et al., 2022; Davenport et al., 2015; Dees et al., 2014, 2018; Eddy et al., 2013; Gibson & Hoefnagels, 2015; Gregory, 2008; Halverson et al., 2011; Johnson et al., 2012; Kong et al., 2016, 2017, 2022; Kummer et al., 2016; MacDonald & Wiley, 2012; MacFadden et al., 2012; Matuk & Uttal, 2011, 2020; Mead, 2009; Meikle & Scott, 2010; Meir et al., 2007; Meisel, 2010; Morabito et al., 2010; Novick & Catley, 2014, 2018; Novick et al., 2011, 2012; Oakley & Pankey, 2008; Oliveira & Cook, 2017; Omland et al., 2008; Pobiner, 2016; Prothero, 2017; Sa’adah et al., 2016; Sandvik, 2008; Schramm & Schmiemann, 2019; Schramm et al., 2021; Seoh et al., 2016; Van Dijk & Reydon, 2010; Wiley, 2010). Although these works on the whole do an admirable job in guiding novices through the challenges of tree thinking, many of them also present incorrect evolutionary views, including (1) the rejection of linear evolutionary imagery and narratives, (2) the rejection of the concept of anagenetic evolution, (3) the rejection of the concept of missing links, (4) the promotion of the flawed concept of collateral ancestors, and (5) the denial that humans evolved from monkeys and apes. Here, I will show that these misconceptions arise from simplistic tree thinking and explain how they can be avoided by adopting what I call lineage thinking. I close by suggesting that teaching can be improved by linking tree thinking explicitly to lineage thinking.
1 Tree Thinking and Lineage Thinking
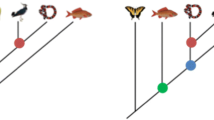
The key to understanding the relationship between tree thinking and lineage thinking as complementary aspects of proper phylogenetic thinking is to distinguish two evolutionary realms that are epistemically and ontologically distinct (Table 1 and Fig. 1). Outside the laboratory, the realm of taxa offers the only observable evidence for evolution. This is the realm of rhinos and daisies and trilobites and everything else visible in the natural world. Since almost all known taxa are related to each other as collateral relatives, this is the branching realm of the products of evolution. In contrast, the invisible process of evolutionary descent with modification that produced all taxa is located in the empirically inaccessible realm of lineages composed of ancestors and their direct descendants. Because very few lineal ancestors of taxa are known, most of these ancestors are hypothetical. Nevertheless, these realms are connected both ontologically and epistemically. When an evolving lineage splits, the shape of evolution changes from linear to branching, as independent lineages embark upon their own phylogenetic trajectories (the complications of reticulate evolution for tree thinking are not the focus of the literature discussed here). Of course, lineage splitting does not sever the ties of descent, so when a lineage splits into two, the topology of evolution is now simultaneously branching and linear. The independently evolving lineages (taxa) are now related as collateral relatives, while the organisms at the leading edge of both lineages are the direct, lineal descendants of linear strings of ancestors stretching back in time (Fig. 1).
A cladogram depicting how we are related to our closest fossil relatives. Of the nine named taxa in the tree, seven represent the tips of diverging lineages. The only two taxa in the tree that are directly ancestral to other taxa are Homo antecessor and Homo heidelbergensis, which are direct lineal ancestors to us and Neanderthals. The other fossil taxa are our collateral relatives, not our ancestors. The topology is based on Parins-Fukuchi et al. (2019). Silhouettes are from phylopic.org
These branching and linear realms are also connected epistemically. By mapping the characters of taxa onto evolutionary trees, researchers make inferences about probable ancestral traits located at different points along lineages (Cunningham et al., 1998; Joy et al., 2016). The pattern of collateral relationships between taxa therefore provides a kind of index for the empirical evidence that is available for inferring lineage evolution. For instance, for the lineage leading to modern humans this index comprises a series of taxa that are increasingly distantly related to Homo sapiens, including Homo habilis, Australopithecus africanus, chimps, gorillas, other primates, and other mammals. Although the realms of taxa and lineages are different facets of the same evolutionary reality, confusion can arise when their distinctive characters are not recognized (see Table 1). As I discuss in detail in my recent book Ancestors in evolutionary biology (Jenner, 2022), by exclusively focusing on taxa and erroneously considering clades to be the central subjects of evolution, Robert O’Hara’s writings promoted a form of simplistic tree thinking that neglects the reality and nature of evolutionary lineages. The resulting fallacy that evolution is irreducibly branched, and that therefore linear evolutionary imagery and narratives should be rejected, was amplified in the influential writings of Stephen Jay Gould. Here, I show how widespread simplistic tree thinking is in the recent educational literature (see Table 2) and how the adoption of lineage thinking can correct these misunderstandings.
2 The Cladistic Blindfold 1: Rejection of Linear Evolutionary Imagery and Narratives
The fundamental flaw of simplistic tree thinking that I call the cladistic blindfold is to focus exclusively on the branching realm of taxa, while overlooking the linear realm of lineages. The rejection of linear evolutionary imagery, like Fig. 2, is an emblematic error that results from donning the cladistic blindfold (Table 2). For example, in his book Evolution: what the fossils say and why it matters, Donald Prothero writes “the tendency to put things into simple linear order is a common metaphor for evolution – and also one of its greatest misperceptions. The iconic image is the classic “ape-to-man” sequence of organisms marching up the evolutionary ladder… This icon of evolution is so familiar that it is parodied endlessly in political cartoons and advertisements… Most people think that this is an accurate representation of evolution. WRONG! Evolution is a bush, not a ladder!” [capitals and italics in original] (Prothero, 2017: 133). Linear evolutionary imagery should indeed be rejected if it inappropriately reflects a scala naturae, in which a series of collateral relatives, like the terminal taxa in a cladogram, are lined up as if they represent an evolutionary lineage. However, the bare-bones geometry of evolutionary descent is linear as well, with a series of organisms or their traits representing successive fossil or hypothetical ancestors in a single evolutionary lineage. Branching evolutionary imagery is needed to capture the divergence of multiple independently evolving lineages produced by lineage splitting (cladogenesis), while reticulate diagrams can capture the fusion of lineages.
A version of the iconic linear march of human evolution. If the images represent hypothetical or fossil ancestors in a lineage rather than collateral relatives, it accurately depicts the bare-bones shape of evolutionary descent (after an original by José-Manuel Benitos, modified by M. Garde, and licensed under a Creative Commons Attribution-Share Alike 3.0 Unported license: https://commons.wikimedia.org/wiki/File:Human_evolution_scheme.svg; last accessed 27 July 2023)
Most of the educational works that I surveyed here promote a false dichotomy between the linear and branching views of evolution (Table 2). In his interesting book on the history of visual metaphors for evolution, David Archibald labeled linear depictions of evolution “Steve Gould’s bane” and blames them for fueling “profound misunderstandings by the general public of how evolution operates” (Archibald, 2014: 19). This view has become so deeply entrenched in the literature that educators judge linear evolutionary diagrams drawn by lay people and students as tell-tale signs of flawed tree thinking (Halverson et al., 2011; Matuk & Uttal, 2011, 2020; Oliveira & Cook, 2017). Indeed, novices often incorrectly line up collateral relatives as if they formed lineages of ancestors and descendants, but none of the authors of the works discussed here offer what to me seems to be an obvious explanation for this. The one thing that many non-experts know about evolution is that it is a process of historical transformation, of things “evolving from” other things, of things “transforming” or “turning” into other things. In the context of this background knowledge, lay people therefore correctly intuit that the fundamental shape of evolution is linear—evolutionary descent tracks the arrow of time. But properly understanding how the linear process of descent relates to the branched relationships between collateral relatives produced by cladogenesis requires the focused training provided by educators. It is worth noting here that this same struggle to come to grips with the dialectics of the linear and branching views of evolution can be traced through the history of evolutionary biology, as scientists grappled with the problem of aligning their theoretical understanding of evolution with the systematic diagrams they were drawing (Jenner, 2022).
Instead of offering the above common-sense explanation for why novices produce linear evolutionary imagery and think about evolution in narrative terms, authors have attempted to ascribe these tendencies to a mixture of cognitive and cultural biases that produce an incorrect but inescapable penchant for linear storytelling (Gould, 1989; Matuk & Uttal, 2011, 2020; O’Hara, 1988; Oikkonen, 2009). The cladistic blindfold can even cause near complete lineage blindness. This is strikingly illustrated by Catley et al. (2010), who asked university students to describe four evolutionary trees. The authors concluded that students who used process phrases like “evolved from,” “evolved into,” and “derived from” in their explanations have a less sophisticated understanding than students who did not use such process phrases. This conclusion is puzzling. Like most non-experts, these students already had the basic understanding that evolution is a process of historical change (they had all taken biology in high school), which lays the foundation for intuitive lineage thinking. Moreover, three of the four trees depicted the evolution of hominins and horses, with fossil taxa deliberately placed in ancestral positions. The other tree was a rendition of Ernst Haeckel’s infamous Pedigree of Man, which includes a series of hypothetical common ancestors along the main trunk. Such trees appropriately invite the use of narrative language to describe the process of evolutionary descent. According to the authors, however, such language is inappropriate because it suggests that evolution can happen by the process of anagenesis, “whereby one species evolves directly into another without any branching events” (Catley et al., 2010: 864). Since they consider such a concept of anagenesis where “one taxon turned into another” to be “spurious” (Catley et al., 2010: 879), they marked down answers that used the language of evolutionary transformation. As we will see, the problem is that Catley et al. and many other authors define anagenesis with respect to the origin of new species. This forces a taxic concept onto the realm of lineages, which inevitably causes problems.
3 The Cladistic Blindfold 2: Rejection of Anagenetic Evolution
Anagenesis receives various definitions in the current literature. For example, in addition to the definition provided by Catley et al., (2010), Hoekstra and Coyne (2007: 1005) define it as “adaptation within lineages,” Rieppel (2013: 163) defines it as “gradual transformation within a species lineage between two cladogenetic events,” and (Ochoterena et al., 2019: 771) define it as “character transformation as a function of time within a lineage.” The consensus underpinning these variations is that anagenesis refers to evolutionary change within lineages. It should therefore be an uncontroversial concept. However, as Table 2 shows, it is rejected in many educational papers, and evolutionary trees with fossils depicted in ancestral positions are therefore deemed inappropriate (Catley & Novick, 2008; Catley et al., 2010; Novick et al., 2011; Schramm et al., 2021). The reason for this is that in these papers anagenesis is invariably defined with respect to the origin of new species, which is contrasted with speciation by cladogenesis. For example, Catley et al., (2010: 864) write that “[a]lthough change within a single species is well documented, one species changing into another species is not.” Linking anagenesis to speciation places an inappropriate taxic constraint on the concept.
Lineages are the fundamental ontological units of evolutionary descent (Table 1) (Burbrink et al., 2022; De Queiroz, 1999, 2005; Padial & De la Riva, 2021). Investigating how taxa relate to lineages is one of the challenging tasks of systematic biology. But no matter if and where we draw diachronic species boundaries along unbranching lineages, these do not signal the cessation of anagenetic evolution. Anagenetic change snakes its way continuously along every evolving lineage in the web of life. When cladogenesis splits a lineage, anagenesis now flows along two diverging lineages. Anagenesis and cladogenesis should not be contrasted as alternative modes of evolution, a conceptual error widely spread by Stephen Jay Gould in both his popular and professional writing (Jenner, 2022). For example, in a technical paper based on his 1987 Presidential Address to the Paleontological Society, Gould (1988: 319) wrote “Professionals, of course, recognize the bushiness of equid and hominid trees, but still view the lone survivors as end-products of a coherent anagenetic sequence within the bush” [italics in original]. And in his popular science book Full House, Gould (1996: 62–63) wrote “Evolution rarely proceeds by the transformation of a single population from one stage the next. Such an evolutionary style, technically called anagenesis, would permit a ladder, a chain, or some similar metaphor of linearity to serve as a proper icon of change. Instead, evolution proceeds by an elaborate and complex series of branching events, or episodes of speciation (technically called cladogenesis, or branch-making)” [italics in original]. This misleading view that the linear and branching views of evolution are somehow in conflict is echoed in much of the educational literature that I surveyed here (Table 2). In reality, they are just different aspects of lineage evolution.
In many educational papers, lineage thinking is compromised by overly taxic thinking. To remove this cladistic blindfold, it is important to keep the ontology of evolution in mind. Brower (2021: 796), for instance, defined lineages as “unbranched series of ancestor and descendant species through time.” But if lineages are the ontological units of evolutionary descent, this definition gets things precisely backwards. The challenge is how to demarcate and define species and supraspecific taxa with respect to evolving lineages. Most of the observable evidence for evolution comes packaged as terminal taxa, so there is an understandable pragmatic temptation to define the invisible realm of lineages in terms of what is empirically accessible. But tying anagenetic evolution exclusively to the origin of species creates a taxic concept that is incommensurate with lineage thinking. Defining it simply as evolution within lineages, anagenesis becomes the beating heart of lineage thinking.
4 The Cladistic Blindfold 3: Rejection of Missing Links
If evolution is descent with modification, then the concept of missing links (transitional forms) expresses the unbroken genealogical continuity of lineages. Ontologically, missing links are equivalent to lineal ancestors, and the concept therefore falls squarely within the purview of lineage thinking. Yet, few evolutionary concepts have been as strongly repudiated in the literature aimed at lay and educational audiences as missing links. What is surprising is that some of the most pointed criticisms have come from paleontologists. Stephen Jay Gould (1998: 202) called missing links a “hoary and clichéd concept,” while Alan Gishlick (2003: 41) and Donald Prothero (2017: 89) dismissed the concept with identical words: “there is no such thing as a missing link.” Henry Gee, who was trained as a paleontologist, explained that he wrote his book The Accidental Species in large part “to explain why terms such as “missing link” encapsulate more than a century of error in thinking about evolution, particularly of human beings. They reinforce a monstrous view of evolution whose function is to cement our own self-regard as the imagined pinnacle of creation” (Gee, 2013: x). And science writer Riley Black, who was resident paleontologist for the 2015 blockbuster movie Jurassic World, included this subhead in a 2018 online article about missing links for the Smithsonian Magazine: “While some still use the term, experts abhor it because it implies that life is a linear hierarchy” (https://www.smithsonianmag.com/science-nature/whats-missing-link-180968327/; last accessed 27 November 2023). One of the experts that Black interviewed for her article was Smithsonian paleoanthropologist Briana Pobiner, who said that “the idea of a “missing link” implies a linear chain of one species evolving into another, evolving into another, and so on,” while in reality evolution “produces a tree-like branching pattern with multiple descendants of an ancestor species existing at the same time, and sometimes even alongside that ancestor species.” Pobiner claims that the “idea that paleontologists (including paleoanthropologists) are searching for “missing links,” a phrase often seen in the media, implies incorrectly that evolution proceeds a linear chain or ladder-like progression” (Pobiner, 2016: 238).
Missing links may be a problematic concept if, like Gee, you see it as a remnant of the pre-evolutionary notion of the scala naturae that is hitched to unsupportable notions, such as predictable evolutionary progress and anthropocentrism. However, to reject it because evolution is branching rather than linear is a form of simplistic tree thinking. It is true that paleontologists do not spend all their time looking for missing links, but this is not because evolutionary descent is not linear. It is because the chance of finding a lineal ancestor to a known taxon is very slim. And yet, it is precisely paleontologists who are sometimes lucky enough to find fossils that are thought to represent a direct link in a lineage leading to a known taxon (Carr et al., 2017; Parins-Fukuchi et al., 2019; Tsai & Fordyce, 2015). Missing links, transitional forms, intermediate forms, and lineal ancestors are real parts of evolving lineages, but they are rarely glimpsed. This is the reason why most of the light that our research sheds on lineage evolution is indirect and refracted back from the study of characters in clades of collateral relatives. But as we will see in the next section, this has led some authors to the peculiar claim that there is a kind of ancestor that can be found in the branching realm of taxa after all.
5 The Cladistic Blindfold 4: The Flawed Concept of Collateral Ancestors
Evolutionists need their sharpest tools in debates with creationists. Because these debates are often marred by accidental and wilful misunderstandings, conceptual precision and clarity are of paramount importance. One of the concepts that some evolutionists have used specifically to explain evolution to creationists is the concept of collateral ancestors (Gishlick, 2003: 41–43; Padian & Angielczyk, 2007; Mead, 2009: 311; Scott, 2009: 190–191; Fitch, 2012: 80; Prothero, 2017: 89). It is usually introduced in an analogy to family relationships, where your lineal ancestors, like your parents, are distinguished from your collateral ancestors, like your uncles. Likewise, the lineal ancestors of a taxon are part of the lineage leading directly to it, while its collateral ancestors are said to share an ancestor with it, without being in its direct line of descent. However, this synonymizes collateral ancestors and collateral relatives. Indeed, Padian and Angielczyk (2007: 211) and Prothero (2017: 89) explicitly equate collateral ancestors with sister taxa! But relatives and ancestors are not the same. The taxic realm of collateral relatives and the realm of lineal ancestors should not be conflated (Table 1).
For example, Fig. 1 depicts how we are related to eight of our closest fossil relatives based on a phylogenetic reconstruction by Parins-Fukuchi et al. (2019). According to this tree, Homo antecessor and Homo heidelbergensis are direct lineal ancestors of both us and Neanderthals. The other fossil taxa are our collateral relatives as they are not directly ancestral to us and instead represent lineages that have diverged from our own ancestral lineage at different points in time.
Using the flawed concept of collateral ancestors is likely to cause confusion in teaching and debates. For example, in their attempts to dismiss fossil evidence for evolution, creationists often correctly point out that Archaeopteryx is not a lineal ancestor of modern birds. In response, some evolutionists then accuse them of naïve, linear scala naturae thinking, while pointing out that Archaeopteryx is in fact a collateral ancestor of modern birds (Gishlick, 2003: 41; Padian & Angielczyk, 2007: 233; Prothero, 2017: 89). This is unlikely to help these authors’ explanations of how evidence from collateral relatives can be used for ancestral state reconstructions. Evolutionists should not forget that most lay people think in terms of linear transformations because they are intuitive lineage thinkers. Non-experts, including creationists, understand that the realms of ancestors and relatives are distinct and are unlikely to be convinced by arguments that conflate them. By importing a taxic concept into the realm of evolving lineages, these authors themselves commit precisely the thought crime of which they accuse creationists. It is therefore of interest here that the founder of phylogenetics already warned lay audiences in 1874 against confusing “the direct descendants with the sidelines,” because “our adversaries often use the mistaken views resulting from this confusion to combat the descent theory” (Haeckel, 1874: 362). The example that Haeckel went on to discuss is the denial that humans evolved from monkeys.
6 The Cladistic Blindfold 5: Humans Did Not Evolve from Monkeys and Apes
A lack of proper lineage thinking by some evolutionists has led to the peculiar situation that they seem to agree with creationists that we did not evolve from monkeys. The denial that we evolved from monkeys and apes is frequently included in online lists of top myths about evolution from respectable outlets (https://www.skeptic.com/downloads/top-10-evolution-myths.pdf; last accessed 27 November 2023; https://www.businessinsider.com/human-evolution-myths-2023-3?r=US&IR=T#myth-survival-of-the-fittest-means-only-the-strongest-survive-3; last accessed 27 November 2023; https://www.pbs.org/wgbh/evolution/library/faq/cat02.html; last accessed 27 November 2023; https://www.bbc.co.uk/news/science-environment-45564594; last accessed 27 November 2023), and it is promoted in literature aimed at educational and lay audiences (Gregory, 2008; Halverson et al., 2011; MacFadden et al., 2012; Meikle & Scott, 2010; Prothero, 2017; Scott, 2009; Smith & Sullivan, 2007). Haeckel (1874: 362) already realized a century and a half ago that many lay people denied our monkey ancestry because they only think in terms of what is familiar to them, namely living species, instead of extinct ancestors. A small but crucial adjustment of language can therefore help correct this misconception: we evolved from extinct monkeys and apes (Jenner, 2018, 2022; Johnson et al., 2012; Meikle & Scott, 2010; Pobiner, 2016). However, this linguistic clarification can be undone by simplistic tree thinking.
Questions about the evolutionary origins of taxa and traits can only be answered with the logic and concepts of lineage thinking. However, a common strategy adopted by tree thinkers is to rephrase these questions in an attempt to answer them with taxic concepts. For example, after Meikle and Scott (2010: 574) correctly point out that some of our ancestors are “fossil apes,” they write that “[i]t is not that humans descended from apes and that apes descended from monkeys; rather, humans and apes share a common ancestor, and it is more recent than the common ancestor they both share with monkeys” (Meikle & Scott, 2010: 575). Reading that some of our ancestors were fossil apes but that we did not evolve from apes is confusing. In their textbook, Baum and Smith (2013: 30) similarly advise that it is prudent to rephrase questions about evolutionary descent in terms of taxic language, so that instead of saying “[h]umans evolved from apes, which evolved from monkeys,” this could be “described using more accurate language, [as] Hominids are a subgroup of apes, and apes are a subgroup of monkeys.” But this is not more accurate language. The specification of these subgroups establishes that non-hominid apes and non-ape monkeys are paraphyletic (a taxonomic group that contains some but not all of the descendants of a specific common ancestor). This is simply a restatement of our evolutionary descent from monkeys and apes in the taxic language of systematics. Before discussing the problem of using taxic language in the realm of lineages, we need to confirm that available evidence indeed shows that some of our ancestors were monkeys and apes.
Figure 3 shows the accepted phylogenetic relationships of humans and their most closely related living primate relatives (Shao et al., 2023). Monkeys and apes are paraphyletic groups. Therefore, the lineage between nodes Z and Y represents our extinct monkey ancestors, while the lineage between nodes X and U represents our extinct ape ancestors. The lineage between nodes Y and X is where our ancestors evolved the characters that distinguish apes from monkeys. The paraphyly of monkeys and apes has been consensus for many decades (Romer, 1954; Shao et al., 2023), so it should be uncontroversial to state that we evolved from monkeys and apes. It is puzzling that this is denied by professional biologists and paleontologists, sometimes in strongly worded statements and in conflict with evidence presented in their own works. For instance, Ryan Gregory (2008: 132), whose paper includes Fig. 3, writes that “no sane biologist” would accept that humans descended from monkeys. Donald Prothero (2017: 374) concludes that people who believe we evolved from monkeys “live in a web of lies.” Surprisingly, Prothero states this 12 pages after showing a tree with an identical topology to Fig. 3 and claiming earlier in his book that “[h]umans still have the genes for the long tails of our monkey ancestors” (Prothero, 2017: 107).
A phylogeny depicting the relationships between humans and our nearest living primate relatives. Clade names discussed in the main text are indicated (modified after Fig. 14a in Gregory, 2008)
Interestingly, when these authors discuss the evolution of birds, they unambiguously conclude that these evolved from, or indeed are, dinosaurs (Gregory, 2008: 131; Scott, 2009: 49; Prothero, 2017: 277). This inconsistency is striking given that the phylogenetic context in these cases is identical. Just as a lineage of dinosaurs evolved into birds, so did a lineage of monkeys give rise to apes and us. Perhaps correct lineage thinking was triggered in the case of the ancestry of birds because it forced these authors to think about fossils (crocodylians, the nearest living relatives of birds, provide a poor starting point for trying to understand bird origins) and adopt a diachronic perspective. But the taxonomic language that we all use to talk about evolution can itself be a hurdle to lineage thinking, especially for novices.
7 The Difficulty of Lineage Thinking with Taxic Concepts
Taxic thinking cannot do explanatory work in the realm of lineages. As O’Hara (1988) rightly pointed out in his original paper on tree thinking, questions about evolutionary descent ask for descriptions and explanations of events, of changes, transformations, and transitions of states. Mere descriptions of states offer no entry into questions about evolutionary origins. What O’Hara did not seem to realize is that by focusing exclusively on the realm of taxa, tree thinking can only offer descriptions of states. Rephrasing “humans evolved from apes and apes evolved from monkeys” as “humans and apes share a common ancestor that is more recent than the common ancestor they share with monkeys” changes a lineage explanation of evolutionary transformation (Calcott, 2009) into a taxic description of relationships. Such taxic descriptions set the necessary context for posing questions about lineage evolution, but due to the lack of a diachronic element they cannot answer them. Without an accompanying tree, one could not even infer from this taxic statement if humans evolved from apes or apes evolved from monkeys. If humans and apes are monophyletic sister taxa, their last common ancestor was neither ape nor human. And if monkeys are the monophyletic sister group to humans and apes, the last common ancestor of all three taxa was not a monkey. But because both monkeys and apes are paraphyletic, we know that segments of our ancestral lineage must have contained extinct primates that deserve to be called monkeys and apes as much as their surviving relatives do.
Of course, a core component of tree thinking is to only accept clades as real systematic entities and to reject supraspecific taxa that are non-monophyletic. From a taxic perspective, this is eminently sensible, but precisely communicating about evolving lineages with the language of monophyletic taxa is difficult. The recognition of paraphyletic taxa that deliberately exclude specific lineages allows us to create evolutionary origin narratives for these lineages in terms of qualitative transformations: some monkeys evolved into apes, which were then no longer monkeys, and some of these apes evolved into humans, who were then no longer apes. The names of paraphyletic taxa provide us with an exclusionary taxic vocabulary that is ideally suited to describing lineage evolution as a flow of changing identities. It is therefore unsurprising that paraphyletic taxa were used so frequently by pre-cladistic evolutionists to talk about evolutionary descent (Jenner, 2022). O’Hara (1992) rightly called paraphyletic taxa “narrative devices” because they allow us to talk about evolving lineages in taxic language. But he and Sandvik (2009: 433) were wrong in condemning this practice for causing “distortions of evolutionary history.” Although recognizing paraphyletic taxa is bad taxonomic practice, they allowed evolutionists to talk and think about lineage evolution in the only language available to them.
Importantly, the use of paraphyletic taxa to convey the sense that evolutionary descent involves a flow of changing identities aligns with lay peoples’ intuitive understanding of how identities can be demarcated along a continuous flow of change in a realm they are intimately familiar with. We recognize that during our lives we go through successive stages, including embryo, fetus, new-born, toddler, adolescent, and adult. These successive identities are exclusive and non-overlapping. We no longer consider an adult to be an adolescent or a toddler, just as we are no longer apes or monkeys. However, this sense of qualitative transformation that is intuitive to non-experts is impossible to convey using the language of monophyletic taxa.
For example, we could say that we are simiiforms and hominoids to specify that we are primates in the clade Simiiformes (monkeys and apes and us) and the subclade Hominoidea (apes and us) (see Fig. 3). But because nested sets of monophyletic taxa form an inclusive hierarchy, logically speaking, we should not say that we evolved from simiiforms or hominoids. Similarly, we do not say that beetles evolved from insects, or that primates evolved from mammals; beetles are insects, and primates are mammals. The taxic language of clades is incommensurate with the narrative language that is appropriate for describing evolutionary descent. Answering the question “what did this taxon evolve from?” with taxic language can only produce a kind of self-referential answers in which evolutionary descent seems to retreat in a preformationist paradox. Did we evolve from primates? No, we are primates. Did we evolve from mammals? No, we are mammals. How can we escape this paradox?
Tree thinkers resort to referring to shared common ancestors. We evolved from a common ancestor we share with other mammals, and more recently we evolved from a common ancestor we share with other primates. But since the identity of these common ancestors is not specified, this does not provide a satisfactory answer to the question of what exactly it was that we evolved from. The solution lies in proper lineage thinking and remembering that clades are what Peter Ax (1985) called “closed descent communities.” Nothing evolves from clades. Evolutionary descent is a lineage-level process, and questions about evolutionary origins should therefore be answered with reference to specific lineages of (often mostly hypothetical) ancestors or what Ax (1985) called stem lineages. It is therefore accurate to say that some of our ancestors were stem primates, stem simiiforms, stem catarrhines, and stem hominoids. However, it is not accurate to say that we evolved from simiiforms, because we are simiiforms (the clade defined by node Z in Fig. 3), but we did evolve from some specific simiiforms, namely the stem catarrhines in our ancestral lineage (the lineage between nodes Z and Y in Fig. 3). This shows that taxic language and the language of stem lineages convey very different aspects about evolution that should not be confused.
Note that by demarcating the successive, discrete segments of our ancestral lineage on the basis of distinct stem lineages, we correctly convey the notion that evolving lineages continuously change identity. This brings lineage thinking into correspondence with lay peoples’ intuitive and correct understanding that identities of time-extended entities, like ourselves, can change over time.
8 Teaching Tree Thinking and Lineage Thinking Together
The works that I have surveyed here perform an essential service to the evolutionary education of non-expert audiences by introducing the fundamentals of tree thinking. However, many of them focus almost exclusively on the branching realm of taxa, while paying insufficient attention to the realm of lineages. This is understandable insofar as almost all empirically accessible evidence for evolution is packaged within taxa. But because most described taxa are the terminal products of evolution, this perspective sidelines the process of evolutionary descent. I have shown that the resulting cladistic blindfold can cause a form of lineage blindness, symptoms of which are the rejection of linear evolutionary imagery, anagenetic evolution, the concept of missing links, the use of narrative language, our monkey and ape ancestry, and the promotion of the illogical concept of collateral ancestors. It is worrying that there are professional researchers who consider branching and linear evolutionary perspectives to be in conflict rather than inextricably linked. For example, MacFadden et al., (2012: 31) write that “instead of a linear sequence in which ancestral species evolve directly into their descendants, the evolutionary tree of horses is bushy.” And in a volume that celebrates the scientific legacy of Stephen Jay Gould, paleoanthropologist Ian Tattersall (2013: 119) approvingly cites Gould’s view that “‘ladders’ (evolution as a continuous sequence of ancestors and descendants) do not represent the path of evolution.” The fact that professional scientists fail to appreciate some of the basic fundamentals of evolutionary biology shows that the cladistic blindfold is a potent conceptual lock.
To improve phylogenetic thinking and teaching requires removing the cladistic blindfold and linking tree thinking explicitly to lineage thinking. The most effective way of doing this is not to start the teaching of evolution by discussing the branching realm of taxa but by first explaining that evolution is a process of descent with modification that happens within lineages of ancestors and descendants. Excellent examples of this are the papers by Baum and Offner (2008) and Brown (2016) and the book by Baum and Smith (2013). By pointing out the distinctive features of the realms of taxa and lineages (Table 1), students can appreciate that different, complementary types of thinking are appropriate for each. Let us celebrate O’Hara’s fruitful phrase of tree thinking, while adding Ax’s stem lineage concept as a much-needed ingredient to the proper teaching of evolution.
References
Ainsworth, S., & Saffer, J. (2013). Can children read evolutionary trees? Merrill-Palmer Quarterly, 59, 221–247.
Archibald, J. D. (2014). Aristotle’s ladder, Darwin’s tree. The evolution of visual metaphors for biological order. Columbia University Press.
Ax, P. (1985). Stem species and the stem lineage concept. Cladistics, 1, 279–287.
Baum, D. A., & Offner, S. (2008). Phylogenics & tree-thinking. The American Biology Teacher, 70, 222–229.
Baum, D. A., & Smith, S. D. (2013). Tree thinking. An introduction to phylogenetic biology. Roberts and Company Publishers.
Baum, D. A., DeWitt Smith, S., & Donovan, S. S. S. (2005). The tree-thinking challenge. Science, 310, 979–980.
Blacquiere, L. D., Fawaz, A., & Hoese, W. J. (2020). Who’s related to whom? Use published phylogenies and make customized tree-thinking assessments. Evolution: Education and Outreach, 13, 20.
Brower, A. V. Z. (2021). A slippery reality: The epistemological shifting sands of tokogeny, phylogeny, lineages and species delimitation. Systematics and Biodiversity, 19, 782–796.
Brown, C. G. (2016). Modeling macroevolution with invented creatures. The American Biology Teacher, 78, 141–148.
Burbrink, F. T., Crother, B. I., Murray, C. M., Smith, B. T., Ruane, S., Myers, E. A., & Pyron, R. A. (2022). Empirical and philosophical problems with the subspecies rank. Ecology and Evolution, 12, e9069.
Calcott, B. (2009). Lineage explanations: Explaining how biological mechanisms change. The British Journal for the Philosophy of Science, 60, 51–78.
Carr, T. D., Varricchio, D. J., Sedlmayr, J. C., Roberts, E. M., & Moore, J. R. (2017). A new tyrannosaur with evidence for anagenesis and crocodile-like facial sensory system. Scientific Reports, 7, 44942.
Catley, K. M., & Novick, L. R. (2008). Seeing the wood for the trees: An analysis of evolutionary diagrams in biology textbooks. BioScience, 58, 976–987.
Catley, K. M., Novick, L. R., & Shade, C. K. (2010). Interpreting evolutionary diagrams: When topology and process conflict. Journal of Research in Science Teaching, 47, 861–882.
Catley, K. M., Phillips, B. C., & Novick, L. R. (2013). Snakes and eels and dogs! Oh, my! Evaluating high school students’ tree-thinking skills: An entry point to understanding evolution. Research in Science Education, 43, 2327–2348.
Cunningham, C. W., Omland, K. E., & Oakley, T. H. (1998). Reconstructing ancestral character states: A critical reappraisal. Trends in Ecology & Evolution, 13, 361–366.
Danos, N., Staab, K. L., & Whitenack, L. B. (2022). The core concepts, competencies, and grand challenges of comparative vertebrate anatomy and morphology. Integrative Organismal Biology, 4, obac019.
Davenport, K. D., Milks, K. J., & Tassell, R. V. (2015). Investigating tree thinking & ancestry with cladograms. The American Biology Teacher, 77, 198–204.
De Queiroz, K. (2005). Different species problems and their resolution. BioEssays, 27, 1263–1269.
Dees, J., Momsen, J. L., Niemi, J., & Montplaisir, L. (2014). Student interpretations of phylogenetic trees in an introductory biology course. CBE-Life Sciences Education, 13, 666–676.
Dees, J., Bussard, C., & Momsen, J. L. (2018). Further effects of phylogenetic tree style on student comprehension in an introductory biology course. CBE-Life Sciences Education, 17, ar17.
Eddy, S. L., Crowe, A. J., Wenderoth, M. P., & Freeman, S. (2013). How should we teach tree-thinking? An experimental test of two hypotheses. Evolution: Education and Outreach, 6, 13.
Fitch, W. M. (2012). The three failures of creationism. Logic, rhetoric, and science. University of California Press.
Gee, H. (2013). The accidental species. Misunderstandings of human evolution. The University of Chicago Press.
Gibson, J. P., & Hoefnagels, M. H. (2015). Correlations between tree thinking and acceptance of evolution in introductory biology students. Evolution: Education and Outreach, 8, 15.
Gishlick, A. D. (2003). Icons of evolution? Why much of what Jonathan Wells writes about evolution is wrong. Pdf from https://ncse.ngo/files/pub/creationism/icons/gishliick_icons_critique_complete.pdf (last accessed on 2 August 2023).
Gould, S. J. (1988). Trends as changes in variance: A new slant on progress and directionality in evolution. Journal of Paleontology, 62, 319–329.
Gould, S. J. (1989). Wonderful life. The Burgess Shale and the nature of history. Penguin Books.
Gould, S. J. (1996). Full house. The spread of excellence from Plato to Darwin. Harmony Books.
Gould, S. J. (1998). Leonardo’s mountain of clams and the diet of worms. Essays on natural history. Harmony Books.
Gregory, T. R. (2008). Understanding evolutionary trees. Evolution: Education and Outreach, 1, 121–137.
Haeckel, E. (1874). Anthropogenie oder Entwicklungsgeschichte der Menschen. Keimes- und Stammes-Geschichte. Verlag von Wilhelm Engelmann.
Halverson, K. L., Pires, C. J., & Abell, S. K. (2011). Exploring the complexity of tree thinking expertise in an undergraduate systematics course. Science Education, 95, 794–823.
Hoekstra, H. E., & Coyne, J. A. (2007). The locus of evolution: Evo devo and the genetics of adaptation. Evolution, 61, 995–1016.
Jenner, R. A. (2022). Ancestors in evolutionary biology. Linear thinking about branching trees. Cambridge University Press.
Jenner, R. A. (2018). Evolution is linear: Debunking life’s little joke. Bioessays, 40. https://doi.org/10.1002/bies.201700196.
Johnson, N. A., Smith, J. J., Pobiner, B., & Schrein, C. (2012). Why are chimps still chimps? The American Biology Teacher, 74, 74–80.
Joy, J. B., Liang, R. H., McCloskey, R. M., Nguyen, T., & Poon, A. F. Y. (2016). Ancestral reconstruction. PLoS Computational Biology, 12, e1004763.
Kong, Y., Thawani, A., Anderson, T., & Pelaez, N. (2017). A model of the use of evolutionary trees (MUET) to inform K-14 biology education. The American Biology Teacher, 79, 81–90.
Kong, Y., Apodaca, J., & Olimpo, J. T. (2022). Implementation and evaluation of the Model of the Use of Evolutionary Trees (MUET) curricular module in an introductory organismal biology course. International Journal of Science Education, 44, 2381–2396.
Kong, Y., Anderson, T., & Pelaez, N. (2016). How to identify and interpret evolutionary tree diagrams. Journal of Biological Education, 50, 395–406.
Kummer, T. A., Whipple, C. J., & Jensen, J. L. (2016). Prevalence and persistence of misconceptions in tree thinking Journal of Microbiology & Biology. Education, 17, 389–398.
MacDonald, T., & Wiley, E. O. (2012). Communicating phylogeny: Evolutionary tree diagrams in museums. Evolution: Education and Outreach, 5, 14–28.
MacFadden, B. J., Oviedo, L. H., Seymour, G. M., & Ellis, S. (2012). Fossil horses, orthogenesis, and communicating evolution in museums. Evolution: Education and Outreach, 5, 29–37.
Matuk, C., & Uttal, D. H. (2011). Narrative spaces in the representation and understanding of evolution. In S. K. Brem, E. M. Evans, & G. M. Sinatra (Eds.), Rosengren KS (pp. 119–144). Oxford University Press.
Matuk, C., & Uttal, D. H. (2020). The effects of invention and recontextualization on representing and reasoning with trees of life. Research in Science Education, 50, 1991–2033.
Mead, L. S. (2009). Transforming our thinking about transitional forms. Evolution: Education and Outreach, 2, 310–314.
Meikle, W. E., & Scott, E. C. (2010). Why are there still monkeys? Evolution: Education and Outreach, 3, 573–575.
Meir, E., Perry, J., Herron, J. C., & Kingsolver, J. (2007). College students’ misconceptions about evolutionary trees. The American Biology Teacher, 69, 71–76.
Meisel, R. P. (2010). Teaching tree-thinking to undergraduate biology students. Evolution: Education and Outreach, 3, 621–628.
Morabito, N. P., Catley, K. M., & Novick, L. R. (2010). Reasoning about evolutionary history: Post-secondary students’ knowledge of most recentcommon ancestry and homoplasy. Journal of Biological Education, 44, 166–174.
Novick, L. R., & Catley, K. M. (2014). When relationships depicted diagrammatically conflict with prior knowledge: An investigation of students’ interpretations of evolutionary trees. Science Education, 98, 269–304.
Novick, L. R., & Catley, K. M. (2018). Teaching tree thinking in an upper level organismal biology course: Testing the effectiveness of a multifaceted curriculum. Journal of Biological Education, 52, 66–78.
Novick, L. R., Shade, C. K., & Catley, K. M. (2011). Linear versus branching depictions of evolutionary history: Implications for diagram design. Topics in Cognitive Science, 3, 536–559.
Novick, L. R., Stull, A. T., & Catley, K. M. (2012). Reading phylogenetic trees: The effects of tree orientation and text processing on comprehension. BioScience, 62, 757–764.
O’Hara, R. J. (1988). Homage to Clio, or, toward an historical philosophy for evolutionary biology. Systematic Zoology, 37, 142–155.
O’Hara, R. J. (1992). Telling the tree: Narrative representation and the study of evolutionary history. Biology and Philosophy, 7, 135–160.
O’Hara, R. J. (1998). Population thinking and tree thinking in systematics. Zoologica Scripta, 26, 323–329.
Oakley, T. H., & Pankey, M. S. (2008). Opening the “Black Box”: the genetic and biochemical basis of eye evolution. Evolution: Education and Outreach, 1, 390–402.
Ochoterena, H., Vrijdaghs, A., Smets, E., & Claßen-Bockhoff, R. (2019). The search for common origin: Homology revisited. Systematic Biology, 68, 767–780.
Oikkonen, V. (2009). Narrating descent: Popular science, evolutionary theory and gender politics. Science as Culture, 18, 1–21.
Oliveira, A. W., & Cook, K. (2017). Student visual communication of evolution. Research in Science Education, 47, 519–538.
Omland, K. E., Cook, L. G., & Crisp, M. D. (2008). Tree thinking for all biology: The problem with reading phylogenies as ladders of progress. BioEssays, 30, 854–867.
Padial, J. M., & De la Riva, I. (2021). A paradigm shift in our view of species drives current trends in biological classification. Biological Reviews of the Cambridge Philosophical Society, 96, 731–751.
Padian, K., & Angielczyk, K. D. (2007). “Transitional forms” versus transtional features. In A. J. Petto & L. R. Godfrey (Eds.), Scientists confront intelligent design and creationism (pp. 197–230). Norton.
Parins-Fukuchi, C., Greiner, E., MacLatchy, L. M., & Fisher, D. C. (2019). Phylogeny, ancestors, and anagenesis in the hominin fossil record. Paleobiology, 45, 378–393.
Pobiner, B. (2016). Accepting, understanding, teaching, and learning (human) evolution: Obstacles and opportunities. American Journal of Physical Anthropology, 159, 232–274.
Prothero, D. R. (2017). Evolution. What the fossils say and why it matters. Columbia University Press.
De Queiroz, K. 1999. The general lineage concept of species and the defining properties of the species category. In: Wilson, R. A. editor. Species. New interdisciplinary essays. MIT Press. p. 49–89.
Rieppel, O. (2013). Biological individuals and natural kinds. Biological Theory, 7, 162–169.
Romer, A. S. (1954). Man and the vertebrates. Penguin Books Ltd.
Sa’adah, S., Hidayat, T., & Sudargo, F. (2016). Undergraduate students’ initial ability in understanding phylogenetic tree. Journal of Physics Conference Series, 824, 012040.
Sandvik, H. (2008). Tree thinking cannot taken for granted: Challenges for teaching phylogenetics. Theory in Biosciences, 127, 45–51.
Sandvik, H. (2009). Anthropocentrisms in cladograms. Biology and Philosophy, 24, 425–440.
Schramm, T., & Schmiemann, P. (2019). Teleological pitfalls in reading evolutionary trees and ways to avoid them. Evolution: Education and Outreach, 12, 20.
Schramm, T., Jose, A., & Schmiemann, P. (2021). Seeing the woods for the trees again: Analyzing evolutionary diagrams in German and US university-level textbooks. Education Sciences, 11, 367.
Scott, E. C. (2009). Evolution vs. creationism. An introduction. University of California Press.
Seoh, K. H. R., Subramaniam, R., & Hoh, Y. K. (2016). How humans evolved according to grade 12 students in Singapore. Journal of Research in Science Teaching, 53, 291–323.
Shao, Y., Zhou, L., Li, F., Zhao, L., Zhang, B. L., Shao, F., Chen, J. W., Chen, C. Y., Bi, X., Zhuang, X. L., & Zhu, H. L. (2023). Phylogenomic analyses provide insights into primate evolution. Science, 380, 913–924.
Smith, C. M., & Sullivan, C. (2007). The top 10 myths about evolution. Prometheus Books.
Tattersall, I. 2013. Stephen J. Gould’s intellectual legacy to anthropology. In: Danieli GA, Minelli A, Pievani T editors. Stephen J. Gould: The scientific legacy. Springer Verlag. p. 115–127.
Tsai, C.-H., & Fordyce, R. E. (2015). Ancestor-descendant relationships in evolution: Origin of the extant pygmy right whale, Caperea marginata. Biology Letters, 11, 20140875–20140875.
Van Dijk, E. M., & Reydon, T. A. C. (2010). A conceptual analysis of evolutionary theory for teacher education. Science & Education, 19, 655–677.
Wiley, E. O. (2010). Why trees are important. Evolution: Education and Outreach, 3, 499–505.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jenner, R.A. Lineage Thinking in Evolutionary Biology: How to Improve the Teaching of Tree Thinking. Sci & Educ (2024). https://doi.org/10.1007/s11191-024-00531-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s11191-024-00531-1