Abstract
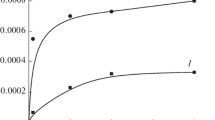
Oxidation of methane with a hydrogen-air mixture at 70 °C and a partial pressure of methane of 30 atm was studied. Water-soluble glutathione-stabilized nanoclusters Aun (n = 18–25) were used as catalysts for this process. Methanol, methyl hydroperoxide (MeOOH), formaldehyde, and a small amount of CO2 were identified as the reaction products. Formation of H2O2 was also revealed; its maximum concentration was 0.5 mmol L−1, and it decreased fivefold in the presence of methane. The study of the reaction kinetics showed that the ratio of initial rates of formation of MeOH, MeOOH, and CH2O was 1: 1: 2. The reaction terminated after 9 h, MeOOH almost completely disappeared, whereas the concentrations of methanol and formaldehyde reached the stationary values of 0.6 and 0.4 mmol L−1, respectively. This phenomenon was observed despite the presence of both the oxidizing agent and the substrate in the reaction zone. Possibly, blockage of active sites by the reaction products took place at a certain time because once the volatile products were removed from the system and the gas phase was renewed, the catalyst showed a stable activity over several cycles. When H2 was excluded from the reaction system, the MeOH yield decreased sixfold, whereas the MeOOH yield tripled after 6 h of reaction. When NADH was used as a hydrogen source, the selectivity with respect to methanol decreased. With the use of quantum chemical calculations, a mechanism for the methane oxidation has been developed. It assumes the existence of the same intermediate as a precursor of all main reaction products.
Similar content being viewed by others
References
C. Hammond, N. Dimitratos, J. A. Lopez-Sanchez, R. L. Jenkins, G. Whiting, S. A. Kondrat, M. H. Ab Rahim, M. M. Forde, A. Thetford, H. Hagen, E. E. Stangland, J. M. Moulijn, S. H. Taylor, D. J. Willock, G. J. Hutchings, ACS Catal., 2013, 3, 8, 1835; DOI: https://doi.org/10.1021/cs400288b.
A. R. Kulkarni, Z. J. Zhao, S. Siahrostami, J. K. Nørskov, F. Studt, Catal. Sci. Technol., 2018, 8, 114; DOI: https://doi.org/10.1039/C7CY01229B.
R. Sharma, H. Poelman, G. B. Marin, V. V. Galvita, Catalysts, 2020, 10, No. 2, 194; DOI: https://doi.org/10.3390/catal10020194.
C. Hammond, M. M. Forde, M. H. Ab Rahim, A. Thetford, Q. He, R. L. Jenkins, N. Dimitratos, J. A. Lopez-Sanchez, N. F. Dummer, D. M. Murphy, A. F. Carley, S. H. Taylor, D. J. Willock, E. E. Stangland, J. Kang, H. Hagen, C. J. Kiely, G. J. Hutchings, Angew. Chem., Int. Ed., 2012, 51, 21, 5129; DOI: https://doi.org/10.1002/anie.201108706.
X. Xuan, L. Wang, B. Yang, C. Fei, T. Y. Yao, W. Liu, Y. Lou, Q. G. Dai, Y. F. Cai, X. M. Cao, Appl. Catal. B, 2021, 285, 119827; DOI: https://doi.org/10.1016/j.apcatb.2020.119827.
L. Tao, I. Lee, M. Sanchez-Sanchez, Catal. Sci. Technol., 2020, 10, 21, 7124; DOI: https://doi.org/10.1039/D0CY01325K.
A. Szecsenyi, G. N. Li, J. Gascon, E. A. Pidko, Chem. Sci., 2018, 9, 6765; DOI: https://doi.org/10.1039/C8SC02376J.
D. Y. Osadchii, A. I. Olivos-Suarez, A. Szecsenyi, G. Li, M. A. Nasalevich, I. A. Dugulan, P. S. Crespo, E. J. M. Hensen, S. L. Veber, M. V. Fedin, G. Sankar, E. A. Pidko, J. Gascon, ACS Catal., 2018, 8, 6, 5542; DOI: https://doi.org/10.1021/acscatal.8b00505.
S. A. Ikbal, C. Colomban, D. W. Zhang, M. Delecluse, T. Brotin, V. Dufaud, J. P. Dutasta, A. B. Sorokin, A. Martinez, Inorg. Chem., 2019, 58, 11, 7220; DOI: https://doi.org/10.1021/acs.inorgchem.9b00199.
A. A. Shteinman, Kinet. Catal., 2020, 61, 339; DOI: https://doi.org/10.1134/S0023158420030180.
X. J. Cui, H. B. Li, Y. Wang, Y. L. Hu, L. Hua, H. Y. Li, X. W. Han, Q. F. Liu, F. Yang, L. M. He, X. Chen, Q. G. Li, J. P. Xiao, D. Deng, X. Bao, Chem., 2018, 4, 1902; DOI: https://doi.org/10.1016/j.chempr.2018.05.006.
L. A. Levchenko, A. P. Sadkov, N. V. Lariontseva, E. M. Koldasheva, A. K. Shilova, A. E. Shilov, J. Inorg. Biochem., 2002, 88 (3–4), 251; DOI: https://doi.org/10.1016/s0162-0134(01)00385-3.
L. A. Levchenko, V. G. Kartsev, A. P. Sadkov, A. F. Shestakov, A. K. Shilova, A. E. Shilov, Dokl. Chem., 2007, 412, 35; DOI: https://doi.org/10.1134/S0012500807020036.
A. F. Shestakov, S. A. Golovanova, N. V. Lariontseva, A. P. Sadkov, V. M. Martynenko, L. A. Levchenko, Russ. Chem. Bull., 2015, 64, 2477; DOI: https://doi.org/10.1007/s11172-015-1180-3.
D. A. Pichugina, N. E. Kuz’menko, A. F. Shestakov, Russ. Chem. Rev., 2015, 84, 1114; DOI: https://doi.org/10.1070/RCR4493.
G. Li, R. Jin, Acc. Chem. Res., 2013, 46, 8, 1749; DOI: https://doi.org/10.1021/ar300213z.
S. J. Freakley, N. Dimitratos, D. J. Willock, S. H. Taylor, C. J. Kiely, G. J. Hutchings, Acc. Chem. Res., 2021, 54, 11, 2614; DOI: https://doi.org/10.1021/acs.accounts.1c00129.
N. J. Gunsalus, A. Koppaka, S. H. Park, S. M. Bischof, B. G. Hashiguchi, R. A. Periana, Chem. Rev., 2017, 117, 13, 8521; DOI: https://doi.org/10.1021/acs.chemrev.6b00739.
M. H. Ab Rahim, M. M. Forde, R. L. Jenkins, C. Hammond, Q. He, N. Dimitratos, J. A. Lopez-Sanchez, A. F. Carley, 5. H. Taylor, D. J. Willock, D. M. Murphy, C. J. Kiely, G. J. Hutchings, Angew. Chem., Int. Ed., 2013, 52, 4, 1280; DOI: https://doi.org/10.1002/anie.201207717.
R. J. Lewis, A. Bara Estaun, N. Agarwal, S. J. Freakley, D. J. Morgan, G. J. Hutchings, Catal. Lett., 2019, 149, 3066; DOI: https://doi.org/10.1007/s10562-019-02876-7.
R. U. McVicker, N. Agarwal, S. J. Freakley, Q. He, S. Althahban, S. H. Taylor, C. J. Kiely, G. J. Hutchings, Catal. Today, 2020, 342, 32; DOI: https://doi.org/10.1016/j.cattod.2018.12.017.
J. K. Edwards, A. F. Carley, A. A. Herzing, C. J. Kiely, G. J. Hutchings, Faraday Discuss., 2008, 138, 225; DOI: https://doi.org/10.1039/B705915A.
M. Okumura, Y. Kitagawa, K. Yamagcuhi, T. Akita, S. Tsubota, M. Haruta, Chem. Lett., 2003, 32, 9, 822; DOI: https://doi.org/10.1246/cl.2003.822.
J. K. Edwards, S. J. Freakley, R. J. Lewis, J. C. Pritchard, G. J. Hutchings, Catal. Today, 2015, 248, 15, 3; DOI: https://doi.org/10.1016/j.cattod.2014.03.011.
P. Landon, P. J. Collier, A. J. Papworth, C. J. Kiely, G. J. Hutchings, Chem. Commun., 2002, 18, 2058; DOI: https://doi.org/10.1039/B205248M.
Y. Yi, L. Wang, G. Li, H. Guo, Catal. Sci. Technol., 2016, 6, 1593; DOI: https://doi.org/10.1039/C5CY01567G.
J. Huang, E. Lima, T. Akita, A. Guzman, C. Qi, T. Takei, M. Haruta, J. Catal., 2011, 278, 1, 8; DOI: https://doi.org/10.1016/j.jcat.2010.11.012.
A. Delparish, S. Kanungo, J. van der Schaaf, M. F. Neira d’Angelo, Catal. Sci. Technol., 2019, 9, 5142; DOI: https://doi.org/10.1039/C9CY01304K.
S. A. Golovanova, A. P. Sadkov, A. F. Shestakov, Kinet. Catal., 2020, 61, 740; DOI: https://doi.org/10.31857/S0453881120040097.
A. P. Kreshkov, Osnovy analiticheskoi khimii. Teoreticheskie osnovy. Kolichestvenniy analiz [Fundamentals of Analytical Chemistry. Theoretical Basis. Quantitative Analysis], Izd-vo Khimiya, Moscow, 1971, 272 pp. (in Russian).
Y. Negishi, K. Nobusada, T. Tsukuda, J. Am. Chem. Soc., 2005, 127, 5261; DOI: https://doi.org/10.1021/ja042218h.
M. Zhu, E. Lanni, N. Garg, M. E. Bier, R. Jin, J. Am. Chem. Soc., 2008, 130, 4, 1138; DOI: https://doi.org/10.1021/ja0782448.
J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865; DOI: https://doi.org/10.1103/PhysRevLett.77.3865.
K. G. Dyall, J. Chem. Phys., 1994, 100, 2118; DOI: https://doi.org/10.1063/1.466508.
D. N. Laikov, Chem. Phys. Lett., 1997, 281, 1–3, 151; DOI: https://doi.org/10.1016/S0009-2614(97)01206-2.
D. N. Laikov, Chem. Phys. Lett., 2005, 416, 1–3, 116; DOI: https://doi.org/10.1016/j.cplett.2005.09.046.
T. C. J. Ovenston, W. T. Rees, Analyst., 1950, 75, 889, 204; DOI: https://doi.org/10.1039/AN9507500204.
J.-P. Lange, V. L. Sushkevich, A. J. Knorpp, J. A. van Bokhoven, Ind. Eng. Chem. Res., 2019, 58, 20, 8674; DOI: https://doi.org/10.1021/acs.iecr.9b01407.
N. Agarwal, S. J. Freakley, R. U. McVicker, S. M. Althahban, N. Dimitratos, Q. He, D. J. Morgan, R. L. Jenkins, D. J. Willock, S. H. Taylor, C. J. Kiely, G. J. Hutchings, Science, 2017, 358, 6360, 223; DOI: https://doi.org/10.1126/science.aan6515.
Z. Wu, R. Jin, ACS Nano, 2009, 3, 7, 2036; DOI: https://doi.org/10.1021/nn9004999.
D. A. Pichugina, N. A. Nikitina, N. E. Kuz’menko, J. Phys. Chem. C, 2020, 124, 5, 3080; DOI: https://doi.org/10.1021/acs.jpcc.9b10286.
N. G. Nikitenko, A. F. Shestakov, Mendeleev Commun., 2017, 27, 2, 144; DOI: https://doi.org/10.1016/j.mencom.2017.03.012.
J. J. Bravo-Suárez, K. K. Bando, T. Fujitani, S. T. Oyama, J. Catal., 2008, 257, 1, 32; DOI: https://doi.org/10.1016/j.jcat.2008.04.004.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 4, pp. 665–674, April, 2022.
This work was performed within the framework of the state task of the Institute of Problems of Chemical Physics of Russian Academy of Sciences (State refistration No. AAAA-A19-119071190045-0).
No human or animal subjects were used in this research.
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Golovanova, S.A., Sadkov, A.P. & Shestakov, A.F. Oxidation of methane to methanol with hydrogen peroxide in situ in the presence of glutathione-stabilized gold nanoclusters under mild conditions. Russ Chem Bull 71, 665–674 (2022). https://doi.org/10.1007/s11172-022-3463-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3463-9