Abstract
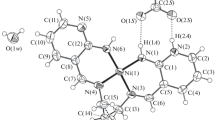
The use of mercury(II) chloride for the synthesis of nido-carborane derivatives with B-O, B-N, and B-S bonds was considered. The involvement of HgCl2 allows one to selectively obtain symmetrically substituted nido-carborane derivatives in high yields. The resulting derivatives can undergo various modification reactions of the organic substituent or be used in the synthesis of metallacarboranes. The structure of the 8,8′-di(dimethyl sulfide)-6,6′-diphenyl derivative of iron(II) bis(dicarbollide) was determined by single-crystal X-ray diffraction.
Similar content being viewed by others
References
R. N. Grimes, Transition Metal Metallacarbaboranes, in Comprehensive Organometallic Chemistry II, Vol. 1, Elsevier, Oxford, 1995, 373.
R. N. Grimes, Coord. Chem. Rev., 2000, 200, 773; DOI: https://doi.org/10.1016/S0010-8545(00)00262-9.
N. S. Hosmane, J. A. Maguire, Metallacarboranes of d- and f-Block Metals, in Comprehensive Organometallic Chemistry III, Vol. 3, Elsevier, Oxford. 2007, 175.
S. V. Timofeev, I. B. Sivaev, E. A. Prikaznova, V. I. Bregadze, J. Organomet. Chem., 2014, 751, 221; DOI: https://doi.org/10.1016/j.jorganchem.2013.08.012.
R. N. Grimes, Carboranes, 3d ed., Academic Press, London, 2016.
O. N. Kazheva, G. G. Aleksandrov, A. V. Kravchenko, V. A. Starodub, G. G. Zhigareva, I. B. Sivaev, V. I. Bregadze, L. I. Buravov, L. V. Titov, O. A. D’yaclienko, Russ. Chem. Bull., 2010, 59, 1137; DOI: https://doi.org/10.1007/s11172-010-0216-y.
S. A. Anufriev, I. B. Sivaev, V. I. Bregadze, Russ. Chem. Bull., 2015, 64, 712; DOI: https://doi.org/10.1007/s11172-015-0924-4.
A. V. Shmal’ko, S. A. Anufriev, A. A. Anisimov, M. Yu. Stogniy, I. B. Sivaev, V. I. Bregadze, Russ. Chem. Bull., 2019, 68, 1239; DOI: https://doi.org/10.1007/s11172-019-2547-7.
A. M. Zinina, S. A. Anufriev, M. A. Derendyaeva, N. A. Knyazeva, N. V. Somov, Yu. B. Malysheva, I. B. Sivaev, I. D. Grishin, Dokl. Chem., 2021, 498, 97; DOI: https://doi.org/10.1134/S0012500821060057.
V. I. Bregadze, S. V. Timofeev, I. B. Sivaev, I. A. Lobanova, Russ. Chem. Rev., 2004, 73, 433; DOI: https://doi.org/10.1070/RC2004v073n05ABEH000868.
F. P. Olsen, M. F. Hawthorne, Inorg. Chem., 1965, 4, 1839; DOI: https://doi.org/10.1021/ic50034a049.
R. H. Pak, R. R. Kane, C. B. Knobler, M. F. Hawthorne, Inorg. Chem., 1994, 33, 5355; DOI: https://doi.org/10.1021/ic00101a031.
G. K. Semin, L. I. Zakharkin, S. I. Kuznetsov, G. G. Zhigareva, E. V. Bryukhova, Russ. J. Gen. Chem., 1998, 68, 919.
E. C. Santos, A. B. Pinkerton, S. A. Kinkead, P. K. Hurlburt, S. A. Jasper, C. W. Sellers, J. C. Huffman, L. J. Todd, Polyhedron, 2000, 19, 1777; DOI: https://doi.org/10.1016/S0277-5387(00)00461-7.
M. Yu. Stogniy, I. B. Sivaev, P. V. Petrovskii, V. I. Bregadze, Russ. J. Gen. Chem., 2012, 82, 91; DOI: https://doi.org/10.1134/S107036321201015X.
D. C. Young, D. V. Howe, M. F. Hawthorne, J. Am. Chem. Soc., 1969, 91, 859; DOI: https://doi.org/10.1021/ja01032a011.
M. M. Vinogradov, I. D. Nesterov, Y. V. Nelubina, A. A. Pavlov, Dalton Trans., 2021, 50, 287; DOI: https://doi.org/10.1039/D0DT03538F.
R. Frank, H. Auer, E. Hey-Hawkins, J. Organomet. Chem., 2013, 747, 217; DOI: https://doi.org/10.1016/j.jorganchem.2013.04.031.
R. Frank, A. K. Adhikari, H. Auer, E. Hey-Hawkins, Chem. Eur. J., 2014, 20, 1440; DOI: https://doi.org/10.1002/chem.201303762.
J. Plešek, T. Jelínek, F. Mareš, S. Heřmánek, Collect. Czech. Chem. Commun., 1993, 58, 1534; DOI: https://doi.org/10.1135/cccc19931534.
P. Řezačova, J. Pokorna, J. Brynda, M. Kožíšek, P. Cígler, M. Lepšík, J. Fanfrlík, J. Řezáč, K. G. Šašková, I. Sieglová, J. Plešek, V. Šícha, B. Grüner, H. Oberwinkler, J. Sedláček, H.-G. Kräusslich, P. Hobza, V. Král, J. Konvalinka, J. Med. Chem., 2009, 52, 7132; DOI: https://doi.org/10.1021/jm9011388.
S. A. Anufriev, I. B. Sivaev, K. Yu. Suponitsky, I. A. Godovikov, V. I. Bregadze, Eur. J. Inorg. Chem., 2017, 4436; DOI: https://doi.org/10.1002/ejic.201700785.
M. Yu. Stogniy, E. N. Abramova, I. A. Lobanova, I. B. Sivaev, V. I. Bragin, P. V. Petrovskii, V. N. Tsupreva, O. V. Sorokina, V. I. Bregadze, Collect. Czech. Chem. Commun., 2007, 72, 1676; DOI: https://doi.org/10.1135/cccc20071676.
M. Yu. Stogniy, I. B. Sivaev, Yu. B. Malysheva, V. I. Bregadze, Vest. Nizhegorodsk. Un-ta im. N. I. Lobachevskogo [Bull. N. I. Lobachevsky Nizhny Novgorod Univ.], 2013, 4, 115 (in Russian).
I. B. Sivaev, M. Yu. Stogniy, Russ. Chem. Bull., 2019, 68, 217; DOI: https://doi.org/10.1007/s11172-019-2379-5.
H. M. Colquhoun, T. J. Greenhough, M. G. H. Wallbridge, J. Chem. Soc., Dalton Trans., 1979, 619; DOI: https://doi.org/10.1039/DT9790000619.
F. Teixidor, J. A. Ayllon, C. Viñas, R. Kivekäs, R. Sillanpää, J. Casabo, J. Organomet. Chem., 1994, 483, 153; DOI: https://doi.org/10.1016/0022-328X(94)87158-2.
K. F. Shaw, B. D. Reid, A. J. Welch, J. Organomet. Chem., 1994, 482, 207; DOI: https://doi.org/10.1016/0022-328X(94)88203-7.
M. Yu. Stogniy, S. A. Erokhina, K. Yu. Suponitsky, A. A. Anisimov, I. B. Sivaev, V. I. Bregadze, New J. Chem., 2018, 42, 17958; DOI: https://doi.org/10.1039/C8NJ04192J.
M. Yu. Stogniy, S. A. Erokhina, A. A. Anisimov, K. Yu. Suponitsky, I. B. Sivaev, V. I. Bregadze, Polyhedron, 2019, 174, 114170; DOI: https://doi.org/10.1016/j.poly.2019.114170.
M. Yu. Stogniy, S. A. Erokhina, I. B. Sivaev, V. I. Bregadze, Phosphorus, Sulfur, Silicon, Related Elements, 2019, 194, 983; DOI: https://doi.org/10.1080/10426507.2019.1631312.
M. Yu. Stogniy, S. A. Erokhina, K. Yu. Suponitsky, A. A. Anisimov, I. A. Godovikov, I. B. Sivaev, V. I. Bregadze, J. Organomet. Chem., 2020, 909, 121111; DOI: https://doi.org/10.1016/j.jorganchem.2020.121111.
M. Yu. Stogniy, S. A. Erokhina, K. Yu. Suponitsky, V. Yu. Markov, I. B. Sivaev, Dalton Trans., 2021, 50, 4967; DOI: https://doi.org/10.1039/D1DT00373A.
S. A. Anufriev, A. V. Shmal’ko, M. Yu. Stogniy, K. Yu. Suponitsky, I. B. Sivaev, Phosphorus, Sulfur, Silicon, Related Elements, 2020, 195, 901; DOI: https://doi.org/10.1080/10426507.2020.1804148.
L. I. Zakharkin, V. N. Kalinin, G. G. Zhigareva, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1979, 28, 2198; DOI: https://doi.org/10.1007/BF00947583.
V. I. Meshcheryakov, P. S. Kitaev, K. A. Lyssenko, Z. A. Starikova, P. V. Petrovskii, Z. Janoušek, M. Corsini, F. Laschi, P. Zanello, A. R. Kudinov, J. Organomet. Chem., 2005, 690, 4745; DOI: https://doi.org/10.1016/j.jorganchem.2005.07.067.
M. Yu. Stogniy, G. S. Kazakov, I. B. Sivaev, V. I. Bregadze, Russ. Chem. Bull., 2013, 62, 699; DOI: https://doi.org/10.1007/s11172-013-0095-0.
G. S. Kazakov, M. Yu. Stogniy, I. B. Sivaev, K. Yu. Suponitsky, I. A. Godovikov, A. D. Kirilin, V. I. Bregadze, J. Organomet. Chem., 2015, 798, 196; DOI: https://doi.org/10.1016/j.jorganchem.2015.05.012.
A. V. Shmal’ko, M. Yu. Stogniy, G. S. Kazakov, S. A. Anufriev, I. B. Sivaev, L. V. Kovalenko, V. I. Bregadze, Dalton Trans., 2015, 44, 9860; DOI: https://doi.org/10.1039/C5DT01293G.
M. Bakardjiev, S. E. Anwar, D. Bavol, Z. Růžičková, B. Grüner, Molecules, 2020, 25, 814; DOI: https://doi.org/10.3390/molecules25040814.
M. Yu. Stogniy, S. A. Anufriev, A. V. Shmal’ko, S. M. Antropov, A. A. Anisimov, K. Yu. Suponitsky, O. A. Filippov, I. B. Sivaev, Dalton Trans., 2021, 50, 2671; DOI: https://doi.org/10.1039/D0DT03857A.
M. Yu. Stogniy, S. A. Erokhina, I. D. Kosenko, A. A. Semioshkin, I. B. Sivaev, Inorganics, 2019, 7, 46; DOI: https://doi.org/10.3390/inorganics7040046.
O. Tutusaus, F. Teixidor, R. Núñez, C. Viñas, R. Sillanpää, R. Kivekäs, J. Organomet. Chem., 2002, 657, 247; DOI: https://doi.org/10.1016/S0022-328X(02)01541-3.
R. Núñez, O. Tutusaus, F. Teixidor, C. Viñas, R. Sillanpää, R. Kivekäs, Chem. Eur. J., 2005, 11, 5637; DOI: https://doi.org/10.1002/chem.200500288.
M. M. Vinogradov, Yu. V. Nelyubina, M. Corsini, F. Fabrizi de Biani, A. R. Kudinov, D. A. Loginov, Eur. J. Inorg. Chem., 2017, 4627; DOI: https://doi.org/10.1002/ejic.201700333.
S. A. Erokhina, M. Yu. Stogniy, K. Yu. Suponitsky, I. D. Kosenko, I. B. Sivaev, V. I. Bregadze, Polyhedron, 2018, 153, 145; DOI: https://doi.org/10.1016/j.poly.2018.07.009.
J. Plešek, B. Štíbr, P. A. Cooke, J. D. Kennedy, T. D. McGrath, M. Thornton-Pett, Acta Cryst. C, 1998, 54, 36; DOI: https://doi.org/10.1107/S0108270197012742.
M. F. Hawthorne, D. C. Young, P. M. Garrett, D. A. Owen, S. G. Schwerin, F. N. Tebbe, P. A. Wegner, J. Am. Chem. Soc., 1968, 90, 862; DOI: https://doi.org/10.1021/ja01006a006.
W. L. F. Armarego, C. L. L. Chai, Purification of Laboratory Chemicals, 6th ed., Butterworth-Heinemann, Burlington, MA, USA, 2009.
APEX2 and SAINT, Bruker AXS Inc., Madison, WI, USA, 2014.
G. M. Sheldrick. Acta Cryst. C, 2015, 71, 3; DOI: https://doi.org/10.1107/S2053229614024218.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences O. M. Nefedov on the occasion of his 90th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 91–101, January, 2022.
This work was financially supported by the Russian Science Foundation (Project No. 19-73-00353). X-ray diffraction and NMR spectroscopic studies were carried out using scientific equipment of the Center for Molecular Structure Studies at the A. N. Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences with financial support of the Ministry of Science and Higher Education of the Russian Federation.
The authors are grateful to K. Yu. Suponitskiy for performing X-ray diffraction studies.
No human or animal subjects were used in this research.
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Stogniy, M.Y., Anufriev, S.A., Bogdanova, E.V. et al. Mercury(II) chloride in the synthesis of nido-carborane derivatives with B-N, B-O, and B-S bonds. Russ Chem Bull 71, 91–101 (2022). https://doi.org/10.1007/s11172-022-3381-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3381-x