Abstract
At first, the preparation and identification of a novel heterogeneous organic–inorganic hybrid acidic nanocatalyst, namely 1,3-(4-pyridyl-1-ium sulfonic acid) (4′-pyridyl-1-ium silica-n-propyl)-propane-dichloride grafted on nano-silica (nano-silica@[DPSSP][Cl]2), have been reported; the identification has been done through a broad range of physicochemical methods comprising FT-IR, SEM, HRTEM, BET, TGA, XRD and EDX techniques. Afterward, nano-silica@[DPSSP][Cl]2 was successfully applied to facilitate the condensation of aryl aldehydes (1 mmol), 2-thiobarbituric acid (2 mmol) and NH4OAc (1.4 mmol) in water solvent (2 mL) at room temperature, such that it provided ten derivatives of pyrido[2,3-d:6,5-d']dipyrimidines (1a–10a) in 5–10 min with yields of 93–99%. Our catalyst furnished the derivatives in very short reaction times and in excellent yields, high atom economy, easy workup and very favorable turnover number (TON) and turnover frequency (TOF) values. In addition, the heterogeneous nature of the catalyst was confirmed and measured using the hot filtration test and its excellent reusability in six runs.
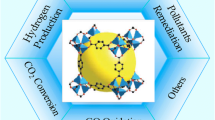
Graphical abstract
















Similar content being viewed by others
Availability of data and materials
All data of this work are reported in the manuscript and supplementary material.
References
Z. Kheilkordi, G. Mohammadi Ziarani, F. Mohajer, A. Badiei, R.S. Varma, J. Inorg. Organomet. Polym. Mater. 33, 1028 (2023)
M. Keshavarz, L.A. Taib, N. Iravani, J. Inorg. Organomet. Polym. Mater. (2023). https://doi.org/10.1007/s10904-023-02747-0
A. Mazraati, M. Setoodehkhah, M. Moradian, J. Inorg. Organomet. Polym. Mater. 32, 143 (2022)
A. Ansari, P. Patel, G. Kumar, H.K. Noor-ul, New J. Chem. 43, 14511 (2019)
M. Shekouhy, R. Kordnezhadian, A. Khalafi-Nezhad, J. Iran. Chem. Soc. 15, 2357 (2018)
Z. Tashrifi, S. Bahadorikhalili, H. Lijan, S. Ansari, H. Hamedifar, M. Mahdavi, New J. Chem. 43, 8930 (2019)
A. Abebe, K.T. Hilawea, Y. Amlaku, B.D. Tamrat, Cogent Chem. 6, 1771832 (2020)
A. Bashti, A.R. Kiasat, Org. Chem. Res. 2, 28 (2016)
A. Bashti, A.R. Kiasat, B. Mokhtari, RSC Adv. 5, 25816 (2015)
A.R. Moosavi-Zare, M.A. Zolfigol, F. Derakhshan-Panah, S. Balalaie, Mol. Catal. 449, 142 (2018)
S. Hosseini, A.R. Kiasat, A. Farhadi, Polycycl. Aromat. Compd. 41, 761 (2021)
A.O. Souza, C.A. Couto-Lima, C.H.R. Catalao, N.N. Santos-Junior, J.F. Santos, M.J.A. Rocha, L.C. Alberici, Neurotoxicology 70, 154 (2019)
T. Rasheed, A.A. Hassan, M. Bilal, T. Hussain, K. Rizwan, Chemosphere 259, 127369 (2020)
T.M. Benedetti, T. Carvalho, D.C. Iwakura, F. Braga, B.R. Vieira, P. Vidinha, J. Gruber, R.M. Torresi, Sol. Energy Mater. Sol. Cells 132, 101 (2015)
S. Sathyamoorthi, M. Kanagaraj, M. Kathiresan, V. Suryanarayanan, D. Velayutham, J. Mater. Chem. A. 4, 4562 (2016)
R. Chen, Z. Jalili, R. Tayebee, RSC Adv. 11, 16359 (2021)
R. Baharfar, S. Peiman, B. Maleki, J. Heterocycl. Chem. 58, 1302 (2021)
R. Taghavi, S. Rostamnia, Chem. Methodol. 6, 639 (2022)
A.R. Moosavi-Zare, H. Goudarziafshar, Z. Jalilian, F. Hosseinabadi, Chem. Methodol. 6, 571 (2022)
A.Z. Hattab, N. Al-Lamia, J.S. Wadib, Eurasian Chem. Commun. 4, 222 (2022)
H. Alinezhad, M. Tajbakhsh, B. Maleki, F. Pourshaban Oushibi, Polycycl. Aromat. Compd. 40, 1485 (2020)
S. Naderi, R. Sandaroos, S. Peiman, B. Maleki, J. Phys. Chem. Solids 180, 111459 (2023)
S.V. Bhaskaruni, S. Maddila, K.K. Gangu, S.B. Jonnalagadda, Arab. J. Chem. 13, 1142 (2020)
D.B. Patel, J.A. Parmar, S.S. Patel, U.J. Naik, H.D. Patel, Curr. Org. Chem. 25, 539 (2021)
M. Taheri, R. Mohebat, Green Chem. Lett. Rev. 13, 165 (2020)
M. Taheri, J. Coord. Chem. 74, 3195 (2021)
G. Brahmachari, K. Nurjamal, I. Karmakar, S. Begam, N. Nayek, B. Mandal, ACS Sustain. Chem. Eng. 3, 9494 (2017)
I.O. Donkor, C.L. Klein, L. Liang, N. Zhu, E. Bradley, A.M. Clark, J. Pharm. Sci. 84, 661 (1995)
E.M. Grivsky, S. Lee, C.W. Sigel, D.S. Duch, C.A. Nichol, J. Med. Chem. 23, 327 (1980)
A. Pastor, R. Alajarin, J.J. Vaquero, J. Alvarez-Builla, M.F. de Casa-Juana, C. Sunkel, J.G. Priego, I. Fonseca, J. Sanz-Aparicio, Tetrahedron 50, 8085 (1994)
V.E. Kolla, A.B. Deyanov, F.Y. Nazmetdinov, Z.N. Kashina, L.P. Drovosekova, Pharm. Chem. J. 27, 635 (1993)
R.K. Rawal, R. Tripathi, S.B. Katti, C. Pannecouque, E. De Clercq, Bioorg. Med. Chem. 15, 3134 (2007)
A.B. Deyanov, R.K. Niyazov, F.Y. Nazmetdinov, B.Y. Syropyatov, V.E. Kolla, M.E. Konshin, Pharm. Chem. J. 25, 248 (1991)
A.R. El-Gazzar, H.N. Hafez, Bioorg. Med. Chem. Lett. 19, 3392 (2009)
A. Monge, V. Martinez-Merino, C. Sanmartin, F.J. Fernandez, M.C. Ochoa, C. Bellver, P. Artigas, E. Fernandez-Alvarez, Eur. J. Med. Chem. 24, 209 (1989)
A. Rosowsky, C.E. Mota, S.F. Queener, J. Heterocycl. Chem. 32, 335 (1995)
J.W. Ellingboe, N.J. Princeton, Chem. Abstr. 124, 176134q (1996)
K. Furukawa, T. Hasegawa, Chem. Abstr. 124, 289568c (1996)
A.D. Broom, J.L. Shim, G.L. Anderson, J. Org. Chem. 41, 1095 (1976)
H. Naeimi, A. Didar, Z. Rashid, J. Iran. Chem. Soc. 14, 377 (2017)
M. Mamaghani, L. Moslemi, A. Badrian, Org. Chem. Res. 3, 1–10 (2018)
H. Naeimi, A. Didar, J. Mol. Struct. 1137, 626 (2017)
H. Naeimi, V. Nejadshafiee, M.R. Islami, Microporous Mesoporous Mater. 227, 23 (2016)
A. Kohzadian, A. Zare, SILICON 12, 1407 (2020)
H. Naeimi, A. Didar, Ultrason. Sonochem. 34, 889 (2017)
H. Naeimi, A. Didar, Z. Rashid, Z. Zahraie, J. Antibiot. 70, 845 (2017)
A. Zare, A. Kohzadian, H. Filian, M.S. Ghoreishi Nezhad, A. Karami, Res. Chem. Intermed. 48, 1631 (2022)
A. Zare, M. Dianat, M.M. Eskandari, New J. Chem. 44, 4736 (2020)
A. Biabani-Ravandi, M. Rezaei, Chem. Eng. J. 184, 141 (2012)
M. Nikoorazm, A. Ghorbani-Choghamarani, M. Khanmoradi, Appl. Organomet. Chem. 30, 705 (2016)
M. Nikoorazm, A. Ghorbani-Choghamarani, N. Noori, B. Tahmasbi, Appl. Organomet. Chem. 30, 843 (2016)
N. Amirmahani, N.O. Mahmoodi, M. Malakootian, A. Pardakhty, N. Seyedi, Res. Chem. Intermed. 46, 4595 (2020)
N. Seyedi, K. Saidi, H. Sheibani, Catal. Lett. 148, 277 (2018)
M.S. Nejad, N. Seyedi, H. Sheibani, S. Behzadi, Mol. Divers. 23, 527 (2019)
Acknowledgements
The authors are grateful to acknowledge the Takin Shimi Sepanta Industries Co., Ilam, Iran.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
S.S.A. synthesized some pyrido[2,3-d:6,5-d']dipyrimidine derivatives (1a–6a) and wrote the manuscript. S.I.S.A. synthesized some pyrido[2,3-d:6,5-d']dipyrimidine derivatives (7a–10a) and helped to edit the manuscript. F.A. identified the structure of some pyrido[2,3-d:6,5-d']dipyrimidine derivatives by 1H NMR, 13CNMR, FT-IR and mass and analyzed their resulting data (1a, 2a and 10a). R.H. optimized the synthesis conditions of pyrido[2,3-d:6,5-d']dipyrimidine derivatives. H.O. identified the structure of the catalyst by means of FE-SEM, HRTEM and EDS analyses. S.B. identified the structure of the catalyst by means of FT-IR and TGA analyses. I.H.K. identified the structure of the catalyst by means of BET and XRD analyses. A.H.R.A. identified the structure of some pyrido[2,3-d:6,5-d']dipyrimidine derivatives by 1H NMR and 13CNMR (4a, 6a and 9a) and analyzed their resulting data. A.H.A. assisted in writing and editing the manuscript. R.F. synthesized the catalyst and helped to edit the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This declaration is not applicable.
Competing interests
This declaration is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdullaev, S.S., Al-Hawary, S.I.S., Al-dolaimy, F. et al. Synthesis, characterization and application of nano-silica@[DPSSP][Cl]2 as a novel organic–inorganic hybrid catalyst for the rapid condensation of aldehyde, ammonium acetate and thiobarbituric acid. Res Chem Intermed 50, 723–743 (2024). https://doi.org/10.1007/s11164-023-05162-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-05162-9