Abstract
A novel nanomaterial was prepared via grafting an organic moiety containing 4,4′-bipyridine scaffold on rice husk-derived nano-silica (RH-SiO2). The nanomaterial namely 1-(Si-pr)-[4,4′-bipyridine]-1,1ʹ-diium hydrogen sulfate grafted on RH-SiO2 (SPBHRS) was characterized by FT-IR, XRD, EDX, FE-SEM and TGA analyses. Afterward, the synthesis of pyrano[2,3-d]pyrimidine-2,4-diones and pyrano[2,3-d]pyrimidine-4-one-2-thiones was performed through the one-pot multi-component reaction of aromatic aldehyde, malononitrile and barbituric acid (or 2-thiobarbituric acid) using SPBHRS as an efficient and recyclable catalyst.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Considering the green chemistry goals, production of ecofriendly and recyclable catalysts from sustainable resources is of great importance. Among the different heterogeneous catalytic supports, silica is usually preferable, due to high surface area, environmentally friendly nature, durability, low cost, easy recycling and simple functionalization by organic components. Silica is the major inorganic constituent of rice husk; extraction of silica from rice husk is a smart and inexpensive alternative to commercial silica, and meantime aids solving rice husk disposal problem faced by rice milling industry. In most rice producing countries, rice husk is usually burnt away causing environmental and health problems [1,2,3,4,5,6].
Nanotechnology allows scientists, physicians, chemists and engineers to work at the molecular levels, and create significant advances in the life sciences to improve human life. The widespread applications of nanomaterials cause the necessity to design new ones with diverse structures [7,8,9,10,11].
Nanomaterials based on silica (as support) not only have been used in catalysis processes [1, 7, 12,13,14,15,16], but also have extensive applications in different industries; e.g., they have been applied as multimodal imaging agent [17], multifunctional additive for enhanced lubrication [18], drug delivery tools for skin cancer (melanoma) treatment [19] and adsorbent [20], and for upcycling waste plastics to fabricate lightweight, waterproof and carbonation resistant cementitious materials [21], degradation of antibiotic in aqueous solution [22] and targeted cancer therapy [23].
Because of the advantages of multi-component reactions, such as high atom economy, flexibility, saving energy and time, selectivity and agreement with green chemistry protocols, they have been utilized as a practical and useful technique for production of complex molecules from simple reactants through a one-pot process [24,25,26,27,28,29].
The compounds containing pyrano-pyrimidine moiety are an attractive group of heterocycles, which have different biological and pharmaceutical activities, e.g., antioxidant [30], antidiabetic [30], antiviral [30], antihypertensive [30], antibacterial [31], antifungal [31], anticancer [32], anti-inflammatory [33], antimicrobial [34] and inhibitor of SARS-CoV-2 [35]. Pyrano[2,3-d]pyrimidine-2,4-diones and pyrano[2,3-d]pyrimidine-4-one-2-thiones could be synthesized through the one-pot multi-component reaction of aromatic aldehyde, malononitrile and barbituric acid (or 2-thiobarbituric acid) using a catalyst [34,35,36,37,38,39,40,41,42,43].
Considering the above issues, a novel nanomaterial based on rice husk-derived nano-silica (RH-SiO2) namely 1-(Si-pr)-[4,4′-bipyridine]-1,1ʹ-diium hydrogen sulfate grafted on RH-SiO2 (SPBHRS) was prepared, and characterized by FT-IR, X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDX), field emission scanning electron microscopy (FE-SEM), thermal gravimetric (TG) and derivative thermal gravimetric (DTG) analyses. It was utilized as an efficient and recoverable catalyst for the synthesis of pyrano[2,3-d]pyrimidine-2,4-diones and pyrano[2,3-d]pyrimidine-4-one-2-thiones from aromatic aldehyde, malononitrile and barbituric acid (or 2-thiobarbituric acid).
Experimental
Materials and instruments
Information of materials and instruments have been given in supplementary material.
Preparation of SPBHRS (Scheme 1)
A mixture of (3-chloropropyl)trimethoxysilane (0.497 g, 2.5 mmol) and 4,4ʹ-bipyridine (0.390 g, 2.5 mmol) in dry toluene (8 mL) was stirred under reflux conditions for 12 h; then, the solvent was distilled under vacuum at 95 °C to provide I. RH-SiO2 (prepared via the reported procedure [44,45,46]) (0.300 g, 5 mmol) and EtOAc (10 mL) were added to compound I, refluxed accompanied with stirring for 15 h, centrifuged, and decanted; the residue was washed by EtOAc (2 × 5 mL), and dried under vacuum at 70 °C to furnish compound II. Finally, II was added slowly to a stirring solution of sulfuric acid (0.245 g, 2.5 mmol) in dry CH2Cl2 (8 mL) at room temperature; the resulting mixture was stirred for 5 h at room temperature and 1 h under reflux conditions; then, it was centrifuged, decanted, washed by CH2Cl2 (3 × 5 mL), and dried under vacuum at 90 °C to afford SPBHRS.
General procedure for the synthesis of pyrano[2,3-d]pyrimidine-2,4-diones and pyrano[2,3-d]pyrimidine-4-one-2-thiones
A mixture of aldehyde (0.5 mmol), malononitrile (0.036 g, 0.55 mmol), barbituric acid or thiobarbituric acid (0.5 mmol) and SPBHRS (0.015 g) in EtOH (5 mL) was stirred under reflux conditions. After completion of the reaction (as observed by TLC), the solvent was distilled, EtOAc (20 mL) was added, stirred for 2 min under reflux conditions, centrifuged, and decanted to isolate the unsolvable SPBHRS (this work was performed two times). The catalyst was washed by EtOAc (2 × 3 mL), dried and used for next run. The resulted EtOAc from the two times decanting was distilled, and the remainder precipitate was recrystallized from EtOH (96%) to afford the pure product.
Note: Selected spectral data and original spectrums of the products have been reported in supplementary material.
Results and discussion
Characterization of SPBHRS
1-(Si-pr)-[4,4′-bipyridine]-1,1ʹ-diium hydrogen sulfate grafted on RH-SiO2 (SPBHRS) was synthesized via the nucleophilic substitution reaction of 4,4ʹ-bipyridine and (3-chloropropyl)trimethoxysilane, then, grafting the obtained compound on rice husk-derived nano-silica, and finally, addition of H2SO4 (Scheme 1). It was characterized by FT-IR, XRD, EDX, FE-SEM, TG and DTG analyses.
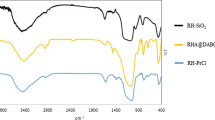
The FT-IR spectrum of SPBHRS (Fig. S1) confirmed presence of the expected bonds and functional groups in its organic and inorganic components. The peaks appeared at 469, 802 and 1094 cm−1 are related to bending modes, symmetric stretching and asymmetric stretching of Si–O bonds in SiO2. The adsorbed H2O molecules on the silica surface gave a peak at 1639 cm−1. The peaks observed at 1416 and 1570 cm−1 are assigned to C = C bonds of the 4,4′-bipyridine moiety. The broad peak at ~ 2781–3733 cm−1 is belong to OH groups of hydrogen sulfate and the adsorbed water on the silica surface. In the FT-IR spectrum of compound I (Fig. S2), the peaks pertained to Si–O bonds of Si–OCH3 moiety (464, 798 and 1100 cm−1), C–O of SiO–CH3 component (1171 cm−1), C = C bond of the 4,4′-bipyridine constituent (1580 cm−1), aliphatic C–H (2927 cm−1) and Si–OH produced by hydrolysis of Si–OCH3 (~ 3315–3691 cm−1) were seen. In the spectrum of compound II (Fig. S3), the bands related to Si–O bonds in SiO2 (470, 803 and 1092 cm−1), C = C bond of the 4,4′-bipyridine component (1417 and 1574 cm−1), aliphatic C–H (2930 cm−1) and OH groups of the adsorbed water on the silica surface (~ 3285–3720 cm−1) were observed. The peak belong to C–O of SiO–CH3 was not seen in the spectrum of compound II, because of bonding I to silica surface and leaving OCH3 (compare the spectrums of I and II). The band related to OH groups of hydrogen sulfate can be seen in the spectrum of SPBHRS, but the spectrum of II did not show this band; this subject confirmed successful performing the reaction of sulfuric acid with compound II to produce SPBHRS.
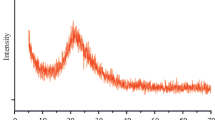
The XRD patterns of SPBHRS (the catalyst) and the reactants used for its synthesis (i.e., RH-SiO2, compound I and compound II) at a range of 2θ = 2–70º are represented in Fig. 1 (the catalyst synthesis is shown in Scheme 1). In the XRD pattern of RH-SiO2, there is a broad peak at 2θ ≈ 15–38º, which is related to amorphous form of SiO2. There are some diffraction lines in the XRD pattern of compound I, which are assigned to its crystalline forms. In the pattern of compound II (which have been synthesized by reacting RH-SiO2 and compound I), observation of the broad peak of amorphous silica and some diffraction lines verified successful grafting compound I on RH-SiO2; this subject was also observed in the catalyst XRD (reducing the diffraction lines in the XRD pattern of SPBHRS in comparison with compound II can be attributed to addition of H2SO4 to compound II to afford SPBHRS). The above explanation approved successful production of SPBHRS.
The EDX spectrum (Fig. 2) showed the peak related to silicon, which is belong to RH-SiO2 and Si-pr constituents of 1-(Si-pr)-[4,4′-bipyridine]-1,1ʹ-diium hydrogen sulfate grafted on RH-SiO2. In the spectrum, the peak pertained to oxygen was observed, which is ascribed to RH-SiO2 and the HSO4− moieties of SPBHRS. The EDX analysis confirmed existence of carbon in the nanomaterial that is attributed to Si-pr and [4,4′-bipyridine]-1,1ʹ-diium components. The presence of the nitrogen related to [4,4′-bipyridine]-1,1ʹ-diium was also approved by the EDX analysis. Existing HSO4− in the SPBHRS structure was verified by observing the peak related to sulfur in the EDX spectrum. Thus, the analysis confirmed presence of all expected elements in the structure of SPBHRS. Moreover, some peaks with low intensity were observed in the spectrum, these can be attributed to the impurities in the rice husk-derived nano-silica, which mentioned in the literature [45, 47].
A sample of SPBHRS was analyzed by FE-SEM to determine the size distribution, particles shapes and surface morphology. The obtained micrographs (Fig. 3) indicated that SPBHRS has porous and irregular shapes, and the particles sizes are less than 100 nm (e.g., 9.71, 14.38, 18.32, 18.52, 20.24, 20.64, 21.06, 24.10, 24.48, 26.12 and 30.21 nm).
Thermal gravimetric analysis of SPBHRS was carried out at a range of 25–600 °C; the corresponding diagrams are illustrated in Fig. 4. According to the diagrams, SPBHRS showed good thermal stability, and decomposed gradually at a range of ~ 210–600 °C with low-speed weight loss. The weight loss below ~ 110 °C can be attributed to the loss of physically adsorbed water and other solvents on the nanomaterial surface. The weight losses at ~ 210–600 °C can be related to the decomposition of 1-(Si-pr)-[4,4′-bipyridine]-1,1ʹ-diium hydrogen sulfate grafted on RH-SiO2 surface and the condensation of the silanol groups. These data confirmed successful grafting the organic component on RH-SiO2 surface, and stability of the nanomaterial up to 210 °C.
Catalytic activity of SPBHRS
Catalytic activity of SPBHRS was tested for the preparation of pyrano[2,3-d]pyrimidine-2,4-diones and pyrano[2,3-d]pyrimidine-4-one-2-thiones. For this purpose, effect of the catalyst loading, solvent and temperature was investigated on the condensation of 4-chlorobenzaldehyde (0.5 mmol), malononitrile (0.55 mmol) and barbituric acid (0.5 mmol), as a model reaction (Scheme 2); the results are summarized in Table 1. The optimal catalyst quantity was found to be 0.015 g (Table 1, entry 3). According to the data acquired from examining the reaction under solvent-free conditions, and in EtOH, EtOH/H2O (1/1), H2O, MeCN and CH2Cl2 (5 mL), the best solvent was EtOH (Table 1, entry 3). Furthermore, the best reaction time and yield were obtained when the reaction was carried out under reflux conditions (Table 1, entry 3).
In continue, to assess the efficiency and generality of SPBHRS for the synthesis of miscellaneous derivatives of pyrano[2,3-d]pyrimidine-2,4-diones and pyrano[2,3-d]pyrimidine-4-one-2-thiones, the reaction of diverse arylaldehydes with malononitrile and barbituric acid (or 2-thiobarbituric acid) was tested in the optimal conditions; the results are given in Table 2. As it can be seen in Table 2, arylaldehydes bearing halogen, electron-releasing and electron-withdrawing substituents on ortho-, meta- and para-positions afforded the relevant products in high yields and relatively short times. The results were satisfactory for both barbituric acid and 2-thiobarbituric acid. According to the results, it can be said SPBHRS was an efficacious and general catalyst for the preparation of the mentioned compounds.
To complete our study on the synthesis and catalytic applicability of 1-(Si-pr)-[4,4′-bipyridine]-1,1ʹ-diium hydrogen sulfate grafted on RH-SiO2 for the preparation of the pyrano-pyrimidine derivatives, its recyclability and reusability were investigated for the preparation of compound 1i (Fig. 5). SPBHRS was reusable for four times with negligible decrement in its catalytic performance.
As it is shown in the reaction mechanism (Scheme 3), hydrogen sulfate of SPBHRS catalyzes the reaction through activation of electrophiles and nucleophiles (steps 1, 3 and 4), assisting removal of H2O (step 2), and helping tautomerization (steps 1, 3 and 5).
Comparison of the reaction conditions and the results of SPBHRS with those of some reported catalysts in the construction of compounds 1a and 1i is illustrated in Table 3. This comparison confirmed superiority of our catalyst with respect to these reported catalysts in at least two of these factors: the reaction temperature, time and yield. Furthermore, in our work, the construction of pyrano[2,3-d]pyrimidine-2,4-diones and pyrano[2,3-d]pyrimidine-4-one-2-thiones has been performed; however, in many of these works, pyrano[2,3-d]pyrimidine-4-one-2-thiones have not been synthesized. The production of nano-silica (as a precursor for the catalyst synthesis) from a sustainable and inexpensive resource (rice husk) is another advantage of our research.
Conclusions
A novel nanomaterial has been prepared based on nano-silica derived from a sustainable and natural resource (i.e., rice husk). It could catalyze the synthesis of pyrano[2,3-d]pyrimidine-2,4-diones and pyrano[2,3-d]pyrimidine-4-one-2-thiones; efficacy, generality, high yields, relatively short reaction times and reusability of the catalyst are some benefits of this catalytic process.
Availability of data and materials
All data of this work have been reported in the manuscript and supplementary material.
References
J. Davarpanah, A.R. Kiasat, RSC Adv. 5, 7986 (2015)
Y. Wang, J. Wu, N. Lin, D. Liu, Z. Liu, H. Lin, Energy 269, 126796 (2023)
J. Davarpanah, M.H. Sayahi, M. Ghahremani, S. Karkhoei, J. Mol. Struct. 1181, 546 (2019)
S. Kamari, F. Ghorbani, Biomass Conv. Bioref. 11, 3001 (2021)
S. Saini, A. Gupta, A.J. Mehta, S. Pramanik, J. Therm. Anal. Calorim. 148, 2335 (2023)
M. S.t Hamzah, M. W. Wildan, Kusmono, E. Suharyadi, J. Asian Ceram. Soc. 11, 178 (2023)
M.A. Bodaghifard, J. Nanostruct. 9, 29 (2019)
K. Safo, H. Noby, M. Matatoshi, H. Naragino, A.H. El-Shazly, Res. Chem. Intermed. 48, 4183 (2022)
H. Teng, Y. Zheng, H. Cao, Q. Huang, J. Xiao, L. Chen, Crit. Rev. Food Sci. Nutr. 63, 378 (2023)
D. Li, Y. Ai, J. Wang, D. Gu, W. Li, Res. Chem. Intermed. 48, 3883 (2022)
Y. Wang, Y. Fei, T. Yang, Z. Luo, Xu. Yanqun, Su. Bin, X. Lin, Nano Today 48, 101749 (2023)
H.S. Oboudatian, J. Safaei-Ghomi, Res. Chem. Intermed. 48, 2069 (2022)
M. Hasanpour Galehban, B. Zeynizadeh, H. Mousavi, J. Mol. Struct. 1271, 134017 (2023)
A. Zare, J. Atashrooz, M.M. Eskandari, Res. Chem. Intermed. 47, 1349 (2021)
N. Nath, S. Chakroborty, P. Panda, K. Pal, Top. Catal. 65, 1706 (2022)
S.Z. Mostashari, A. Fallah Shojaei, K. Tabatabaeian, H. Kefayati, S. Shariati, Res. Chem. Intermed. 48, 669 (2022)
A. Najdian, M. Amanlou, D. Beiki, A. Bitarafan-Rajabi, M. Mirzaei, M. Shafiee Ardestani, Bioorg. Chem. 125, 105827 (2022)
S. Xiong, B. Zhang, S. Luo, H. Wu, Z. Zhang, Friction 9, 239 (2021)
H.T. Trinh, S. Mohanan, D. Radhakrishnan, S. Tiburcius, J.-H. Yang, N.M. Verrills, A. Karakoti, A. Vinu, Emerg. Mater. 4, 1067 (2021)
H. Li, X. Chen, D. Shen, F. Wu, R. Pleixats, J. Pan, Nanoscale 13, 15998 (2021)
A. Al-Mansour, Y. Dai, C. Xu, R. Yang, J. Lu, Y. Peng, J. Wang, Q. Lv, Q. Zeng, Mater. Today Sustain. 21, 100325 (2023)
M. Esmati, A. Allahresani, A. Naghizadeh, Res. Chem. Intermed. 47, 1447 (2021)
Y. Kazemi, S. Dehghani, F. Soltani, K. Abnous, Mona Alibolandi, S.M. Taghdisi, M. Ramezani, Nanomed. Nanotechnol. Biol. Med. 45, 102588 (2022)
F. Ghadirian, A.R. Momeni, J. Albadi, Iran. J. Catal. 10, 155 (2020)
L.-N. Dong, S.-Z. Zhang, W.-L. Zhang, Y. Dong, L.-P. Mo, Z.-H. Zhang, Res. Chem. Intermed. 48, 1249 (2022)
A. Khalafi-Nezhad, M. Divar, F. Panahi, RSC Adv. 5, 2223 (2015)
A.P. Katariya, P.D. Shirsath, H. Narode, P.B. Gaikwad, G.G. Kadam, M.V. Katariya, S.U. Deshmukh, Mol. Divers., 27, in press (2023), doi: https://doi.org/10.1007/s11030-022-10572-9
A. Zare, A. Kohzadian, H. Filian, M.S. Ghoreishi Nezhad, A. Karami, Res. Chem. Intermed. 48, 1631 (2022)
A. Zare, A. Kohzadian, Z. Abshirini, S.S. Sajadikhah, J. Phipps, M. Benamarad, M.H. Beyzavi, New J. Chem. 43, 2247 (2019)
A.Y. El-Khateeb, S.E. Hamed, K.M. Elattar, RSC Adv. 12, 11808 (2022)
S. Maddila, K. Nagaraju, S.B. Jonnalagadda, Chem. Data Collect. 28, 100486 (2020)
N.E.A.A. El-Sattar, K. El-Adl, M.A. El-Hashash, S.A. Salama, M.M. Elhady, Bioorg. Chem. 115, 105186 (2021)
M.D. Naik, Y.D. Bodke, J. Prashantha, J.K. Naik, J. Chem. Sci. 133, 17 (2021)
A.V. Chate, R.M. Dongre, M.K. Khaire, G.M. Bondle, J.N. Sangshetti, M. Damale, Res. Chem. Intermed. 44, 6119 (2018)
A.R. Nesaragi, R.R. Kamble, S.R. Hoolageri, A. Mavazzan, S.F. Madar, A. Anand, S.D. Joshi, Appl. Organomet. Chem. 36, e6469 (2022)
M. Biglari, F. Shirini, N.O. Mahmoodi, M. Zabihzadeh, M. Safarpoor Nikoo Langarudi, M. Alipour Khoshdel, Polycycl. Aromat. Compd. 42, 1452 (2022)
S. Balalaie, S. Abdolmohammadi, H.R. Bijanzadeh, A. Mohammad Amani, Mol. Divers. 12, 85 (2008)
G. Mohammadi Ziarani, S. Faramarzi, S. Asadi, A. Badiei, R. Bazl, M. Amanlou, Daru J. Pharm. Sci. 21, 3 (2013)
S. Sorkhabi, R. Mozafari, M. Ghadermazi, Appl. Organomet. Chem. 35, e6225 (2021)
N. Daneshvar, M. Nasiri, M. Shirzad, M. Safarpoor Nikoo Langarudi, F. Shirini, H. Tajik, New J. Chem. 42, 9744 (2018)
I. Mousazadeh Moghaddampour, F. Shirini, M. Safarpoor Nikoo Langarudi, Polycycl. Aromat. Compd. 42, 2471 (2022)
M. Biglari, F. Shirini, N.O. Mahmoodi, M. Zabihzadeh, M. Mashhadinezhad, J. Mol. Struct. 1205, 127652 (2020)
J. Albadi, A. Mansournezhad, T. Sadeghi, Res. Chem. Intermed. 41, 8317 (2015)
D. Datta, G. Halder, J. Polym. Environ. 27, 710 (2019)
A.R. Alvee, R. Malinda, A.M. Akbar, R.D. Ashar, C. Rahmawati, T. Alomayri, A. Raza, F.U.A. Shaikh, Case Stud. Constr. Mater. 16, e00792 (2022)
A. Jyoti, R.K. Singh, N. Kumar, A.K. Aman, M. Kar, Mater. Sci. Eng. B 263, 114871 (2021)
J.H. Lee, J.H. Kwon, J.-W. Lee, H.-S. Lee, J.H. Chang, B.-I. Sang, J. Ind. Eng. Chem. 50, 79 (2017)
Acknowledgements
The authors thank Research Council of Payame Noor University for the support of this work.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
AZ has designed the research project, wrote the main manuscript text, and interpreted the spectra and analyses. EI has performed the experimental works. All authors have reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
This declaration is not applicable.
Ethical approval
This declaration is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zare, A., Izadi, E. Preparation of a novel nanocatalyst based on rice husk-derived nano-silica and its application for the synthesis of pyrano[2,3-d]pyrimidine-2,4-diones and pyrano[2,3-d]pyrimidine-4-one-2-thiones. Res Chem Intermed 49, 2919–2931 (2023). https://doi.org/10.1007/s11164-023-05021-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-05021-7