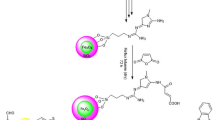
Abstract
A novel and reusable nanomagnetic catalyst, Fe2O3@SiO2/In2O3, was synthesized by a facile chemical approach in three successive steps. The nanocatalyst was characterized by FT-IR, XRD, SEM, EDX, TEM, and VSM. The XRD pattern displays the characteristic peaks of Fe2O3 and SiO2, accompanied by new peaks assigned to different planes of In2O3 that confirm the formation of In2O3 on the surface of Fe2O3@SiO2 core/shell spindles. The TEM micrographs show spindle-like particles of Fe2O3 covered with SiO2 shell, and the In2O3 nanoparticles in an average diameter of 20 nm are hung on the surface of the Fe2O3@SiO2. The nanomagnetic catalyst Fe2O3@SiO2/In2O3 was used for the transformation of the (4-nitrophenyl)-1-phenyl-1H-pyrazole-5-amine, and chalcones derivatives, into valuable azomethine compounds of 3-(substituted)-1-(pyridine-2-yl)allylidene)-3-(4-nitrophenyl)-1-phenyl-1H-pyrazole-5-amine with high rate and efficient catalyst recovery. The yield obtained through the catalytic route reached 90–95% in shorter reaction times compared with uncatalyzed reaction method.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of low-cost and recyclable catalysts is attractive to whom are involved in the green synthesis of organic compounds. By using catalysts, one can reduce the reaction temperature, and the amount of reagent-based waste, but enhance the selectivity of the reaction and avoid undesired side products, to quire a green technology [1]. Nanomaterials are used as catalysts in organic transformations to improve the selectivity and yield of various organic reactions and verify the aspects of green organic synthesis. This is due to their unique surface features, which are the essential difference from those of corresponding bulk materials [2, 3]. In nanocatalysts, the high surface area/volume ratio implies a large number of active sites that participate in the reactions. Nanocomposites open a wide spectrum of desirable synergistic and complementary effects as heterogeneous catalysts for various organic transformations. Metal oxides based nanocomposites are used in heterogeneous catalysis of different organic transformations, due to their optimum porous size, extensive surface area, and large thermal stability [4, 5]. The nano-mixed oxides attracted attention because they are more efficient than their single and bulk counterparts [6]. Recently, magnetic nanoparticles have also received considerable attention owing to their superparamagnetic properties, high adsorption capacities, and high surface area. Iron oxide nanoparticles (NPs) especially hematite (Fe2O3) are used in catalysts, photoelectrochemical cells, sensors, data storage materials, pigments, and fine ceramics. It has a rhombohedral and centered hexagonal structure with a close-packed oxygen lattice [7]. They could be isolated from the reaction medium by the effect of an external magnetic field and can be reused for further reactions [8,9,10].
Silica nanoparticles were used as nano-adsorbents because it is low-cost and reusable, have tunable pore diameters, excellent chemical, and are thermally stable. It has structural characteristics, a large surface area, high selectivity for organic pollutants, and ease of surface modification [11,12,13].
Indium oxide (In2O3) is an n-type semiconductor with a band gap of around 3.6 eV at room temperature. Recently, it has received much attention because of its applications in many fields such as field-emission displays, lithium-ion batteries, solar cells, nanoscale biosensors, optoelectronics, gas sensor, and photocatalysis [14].
The synthesis of nitrogen-containing aromatic heterocyclic 5-amino-pyrazoles-based compounds, as azomethine compounds by simple and efficient routes, is demanded. Azomethines, on the other hand, are commonly utilized organic compounds, in analytical, biological, and inorganic chemistry, due to their physiological, pharmacological, and biological actions [15,16,17,18,19].
Designing and developing ideal catalysts is a very important aspect of green chemistry, so a great interest was devoted to developing non-toxic, environmentally acceptable, low-cost, and recyclable catalysts to provide high productivity under mild reaction conditions. Relevant nanomaterials have a lot of promise to be employed as catalysts for organic transformations [20, 21]. Therefore, a reasonably cheaper, highly recyclable, and efficient catalyst is required [22,23,24]. In this paper, a novel magnetic nanocatalyst, Fe2O3@SiO2/In2O3, has been synthesized for the first time by a facile chemical approach in three successive steps and characterized by different analytical techniques. It was then applied as a catalyst to obtain a fast and efficient cyclo-condensation reaction of 5-amino pyrazole with chalcones derivatives to give the corresponding important 5-amino-pyrazoles based azomethine derivatives. The catalytic reaction rate and the yield of products were determined and compared with those obtained from the uncatalyzed route of reactions.
Experimental
Materials
FeCl3.6H2O, polyvinylpyrrolidone (PVP), anhydrous sodium acetate (NaAC), ethylenediamine, and tetraethyl orthosilicate (TEOS) were purchased from Sigma-Aldrich. Ethanol, indium nitrate hydrate, and urea were obtained from Sigma- Aldrich. Unless otherwise stated, all reagents of analytical grade were purchased from Sigma-Aldrich and were used without further purification.
Instrumentation
The crystalline structure of synthesized nanoparticles was examined by X-ray powder diffraction (XRD) with a GNR APD 2000 PRO diffractometer. The X-ray beam was nickel-filtered Cu Kα (λ = 1.5405 Å) radiation operating at 40 kV and 30 mA and the scanning speed was 0.03 degree/1 s. The morphology of the nanocomposites was obtained by a scanning electron microscope (SEM), (JEOL Japan JSM IT-100) and transmission electron microscope (200 kV TEM / FEG TEM, Japan, (JEM-2100), JEOL). The energy dispersive X-ray spectroscopy (EDX) IT100LA operating at an accelerating voltage of 20.00 keV is attached to the scanning electron microscope. Magnetic properties were measured by a vibrating sample magnetometer (VSM, Lake Shore, 7410 model) at room temperature. The FT-IR spectra were recorded by FT-IR-4100 (JASCO, Japan) spectrophotometer using KBr pellets in the wavenumber range 4000–400 cm−1 with a resolution of 2 cm−1. The nuclear magnetic resonance (1HNMR) spectra were recorded on a Bruker AC spectrometer (400 MHz) at 25 °C in d6-DMSO with TMS as an internal standard, and chemical shifts are reported in parts per million as d values; (13CNMR) was set at 101 MHz. Mass spectra were measured on a Finnigan MAT 8222 EX electron impact mass spectrometer (EIMS) at 70 eV. The melting points were measured without corrections on a Gallenkamp melting point apparatus. Elemental analyses for C, H, and N were also carried out at the regional center for mycology and biotechnology, and the values were found to be within ± 0.4% of the theoretical ones unless otherwise indicated.
Synthesis of Fe2O3 spindles
Fe2O3 spindles were synthesized from an aqueous solution. 2 g of FeCl3.6H2O and 1 g of PVP were dissolved with stirring in 30 mL of water until a clear solution was developed. 2 g of anhydrous sodium acetate followed by 7 mL of ethylenediamine were added. The mixture was then sealed in an autoclave and heated at 200 °C for 10 h. The Fe2O3 spindles were collected, washed perfectly with water, and dried [25] Fig. 1.
Synthesis of Fe2O3@SiO2 core/shell
2 g of Fe2O3 spindles were first dispersed in a solution consisting of 400 mL of ethanol and 100 mL of water and sonicated till dispersion. 10 mL of ammonia were then added to the suspension followed by 6 mL of TEOS under constant magnetic stirring. The mixture was left for 4 h at 25 °C. The formed Fe2O3@SiO2 core/shell was obtained by centrifugation and washed with ethanol and water repeatedly [26] Fig. 1.
Synthesis of Fe2O3@SiO2/In2O3 nanocomposite
This nanocomposite was prepared as follows. 1 g of indium nitrate hydrate, 1 g of urea, and 0.5 g of Fe2O3@SiO2 were mixed in 200 mL of ethyl alcohol and stirred for 40 min. The mixture was transferred into a 100-mL Teflon-lined stainless steel autoclave and maintained at 180 °C for 2 h, followed by 160 °C for 8 h. After that, the autoclave was left to cool to ambient temperature. The obtained material was centrifuged and washed with water and absolute ethanol. It was then dried at 80 °C for 12 h and calcined in air at 400 °C for 2 h. [27] Fig. 1.
Synthesis of 3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-5-amine (1)
An aqueous solution of sodium cyanide (2.8 g, 57 mmol) in 10 ml of water was added dropwise to an ethanolic solution of 2-bromo-1-(4-nitrophenyl)ethan-1-one (4.84 g, 20 mmol). The reaction mixture was kept for four hours with stirring at room temperature, the reaction mixture was diluted with water (35 mL) and again kept for an additional four hours with stirring. The solution was filtered and the filtrate was acidified with acetic acid (30%) till pH 5–6, cooled and the formed solid was filtered to give 3-(4-nitrophenyl)-3-oxopropanenitrile.
Phenyl hydrazine (0.76 g, 7 mmol) was added to (1.29 g, 6.8 mmol) of 3-(4-nitrophenyl)-3-oxopropanenitrile in absolute ethanol (5 mL) and acetic acid (0.2 mL). The reaction mixture was refluxed for 10 h, and the excess solvent was removed under reduced pressure. The crude product was crystallized from ethanol to give the compound 1 in Fig. 2.
Dark brown color; Yield: 1.23 g (65%); m.p = 160–163 oC: IR: ν/cm−1: 3318 (NH2), 3074 (CH-Aro.), 1599 (C = N); 1H NMR (400 MHz, DMSO) δ/ppm: 5.22 (s, 2H, NH2 exchangeable with D2O), 5.58 (s, 1H, CH of pyrazole), 7.22–8.36 (m, 9H, Ar–H). 13C NMR (101 MHz, DMSO) δ/ppm: 86.48 (C4 of pyrazole), 146.79 (C3 of pyrazole), 148.46 (C5 of pyrazole), 125.24, 125.35, 126.85, 128.43, 129.41, 136.49, 138.63, 147.56 (Carom.). MS (EI) m/z: calculated for C15H12N4O2 [M] +, 280.28; found, 280.52.
Synthesis of substituted chalcones (2a-c)
The investigated chalcones (2a-b) are synthesized according to Claisen–Schmidt condensation [28]. Equimolar concentrations (1.21 g, 10 mmol) of 1-(pyridine-2-yl) ethanone and respective aldehydes (10 mmol) were mixed and dissolved in a water–ethanol mixture (30%, v/v) (30 mL). To this solution, 20-mL aqueous sodium hydroxide solution (20%) was added dropwise with stirring, and the reaction mixture was kept under stirring overnight at room temperature. Completion of the reaction was identified by observing thin layer chromatography (TLC) on pre-coated SiO2 gel plates. After completion of the reaction, the reaction mixture was poured into crushed ice, and acidified with dilute HCl till the chalcones were precipitated out as solid. The solid separated was filtered off and dried. It was purified by recrystallization from ethanol Fig. 3. Reaction progress was monitored by TLC using benzene/ethyl acetate (3/1 by volume) as eluent. The 5-(4-(dimethylamino)phenyl)-1-(pyridine-2-yl)penta-2,4-dien-1-one (2c) was prepared according to the previously reported method [29]. The obtained chalcones are:
-
(2a): 3-(Naphthalen-2-yl)-1-(pyridin-2-yl)prop-2-en-1-one
Faint yellow color; Yield: 2.07 g (80%); m. p = 195–198 ºC: IR: ν/cm−1: 3050 (CH-Aro.), 1575 (C = N); 1679 (C = O), 1H NMR (400 MHz, d6- DMSO) δ/ppm: 7.11 (d, 1H, J = 7.05, Hz CH = CH), 7.27(d, 1H, J = 7.32, CH = CH), 8.81–8.76 (m, 4H, Py-H), 7.39–8.65 (m, 7H, Ar–H), MS (EI) m/z: calcd. for C18H13NO [M] +, 259.10; found, other fragments 259.3, 241.1,230.1,181.05,152.05,106.1, 78.05 (base beak).
-
(2b): 3-(Anthracen-2-yl)-1-(pyridine-2-yl) prop-2-en-1-one
Yellow orange color; Yield: 2.32 g (75%); mp = 145-1480C: IR: ν/cm−1: 3054 (CH-Aro.), 1574 (C = N); 1665 (C = O), 1H NMR (400 MHz, d6- DMSO) δ/ppm: 7.58(d, 1H, J = 7.62, CH = CH), 7.60 (d, 1H, J = 7.68, CH = CH), 7.76–8.77 (d, 4H, Py-H), 7.61–8.71 (m, 9H, Ar–H), MS (EI) m/z: calcd. for C22H15NO [M] +, 309.36; found, other fragments 309.10, 280.1,231.05, 203.05 (base beak),176.05, 78.05.
-
(2c): 5-(4-(Dimethylamino phenyl)-1-(pyridine-2-yl) penta-2,4-dien-1-one
Red color; Yield: 2.17g (78%); m.p = 221-223°C: IR: ν/cm-1: 3054 (CH-Aro.), 1574 (C=N); 1665 (C=O), 1H NMR (400 MHz, d6- DMSO) δ/ppm: 7.58(d, 1H, J = 7.55, CH=CH), 7.60 (d, 1H, J = 7.66, CH=CH), 7.76- 8.77(d, 4H, Py-H), 7.61-8.71 (m, 9H, Ar-H), MS (EI) m/z: calcd. for C22H15NO [M] +, 278.35; found, 278.28, other fragments 257, 224, 178, 145, 97, 81, 69 (base beak) [29].
Synthesis of 3-(substituted)-1-(pyridine-2-yl)allylidene)-3-(4-nitrophenyl)-1-phenyl-1H-pyrazole-5-amine derivatives (3a-c)
Conventional (uncatalyzed) route
2.8 g (10 mmol) of 3-(4-nitrophenyl)-1-phenyl-1H-pyrazole-5-amine 1 and 2.59 or 3.09 or 2.78 g (10 mmol) of chalcones 2a-c were dissolved in 20 mL of DMF, then 4–5 drops of concentrated H2SO4 were added. The mixture was refluxed at 110 °C for 5–8 h. The progress of the reaction was monitored by TLC. The reaction mixture was then cooled, poured on crushed ice, and stirred. The solid material produced was collected by filtration, purified by silica gel chromatography (silica gel 60–120 mesh, eluent 20% ethyl acetate/benzene) Fig. 4.[30, 31].
Catalyzed route
Equimolar concentrations (10 mmol) of 3-(4-nitrophenyl)-1-phenyl-1H-pyrazole-5-amine 1 and synthesized chalcones 2a-c were dissolved in 20-mL DMF, then 0.1 g of the nanocatalyst Fe2O3@SiO2/In2O3 was added and stirred. The reaction mixture was refluxed at 110 °C for 20–30 min. The completion of the reactions was indicated by TLC. The reaction mixture was allowed to cool to ambient temperature, and diluted with water. The catalyst was then filtered washed with ethanol and dried in air at ambient temperature for more than 2 h for subsequent reuse. The filtrate was extracted using ethyl acetate (2 × 20 mL). The combined organic layers were dried over anhydrous Na2SO4, and the organic solvent was evaporated by a rotatory evaporator. The crude residue was purified by column chromatography (silica gel 60–120 mesh, with an eluent of 20% ethyl acetate/benzene mixture), Fig. 4.
-
(3a): N-(-3-(naphthalen-1-yl)-1-(pyridin-2-yl)allylidene)-3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-5-amine
Reddish brown color; Yield: 4.01 g (77%); m.p = 78–80 0C: IR: ν/cm−1: 2919 (CH-Aro.),1673 (C=N pyridine ring), 1590 (C=N imine), 1510 (C=Cchain); 1H NMR (400 MHz, d6- DMSO) δ/ppm: 7.21(s, 1H, Pyrazole- H4), 7.62 (d, 1H, J = 7.77 Hz, CH=CH), 7.75 (d, 1H, J = 7.69 Hz, CH=CH), 7.59–8.79 (m, 4H, J = 7.59, Py-H), 7.21–8.33 (m, 17H, Ar–H). 13C NMR (101 MHz, DMSO) δ/ppm: 94.3 (C4 of pyrazole ring), 144.8 (C3 of pyrazole ring), 152.3 (C5 of pyrazole ring), 129.5 (CH=CH), 139.3 (CH=CH), 156.2 (C=N imine), 157.78 (C=N pyridine ring), 123.4, 124.1, 124.5, 125.1, 125.4, 126.2, 126.8, 127.1, 127.3, 128.2, 128.5, 128.8, 129.8, 131.3, 132,3, 133.6, 136.9, 136.8, 137.3, 138.2, 138.57, 139.3, 139.9, 140.4 (Carom.); MS (EI) m/z: calculated for C33H23N5O2 [M]+, 521.56; found, M+ + 1 = 522.79, other fragments 479.75,385.67,266.78, 244, 140.17, 111.11 (base beak), 76.49.
-
(3b): N-(3-(anthracen-1-yl)-1-(pyridin-2-yl)allylidene)-3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-5-amine
Dark brown color; Yield: 2.29 g (75%); m.p = 185–190 0C: IR: ν/cm−1: 2920 (CH-Aro.),1638 (C=N pyridine ring), 1517 (C=N imine), 1458 (C=Cchain); 1H NMR (400 MHz, d6- DMSO) δ/ppm: 7.42 (s, 1H, Pyrazole-H4), 7.57 (d, 1H, J = 7.48 Hz, CH=CH), 7.71 (d, 1H, J = 7.82 Hz, CH=CH), 7.60–8.83 (m, 4H, Py-H), 7.42–8.56 (m, 19H, Ar–H). 13C NMR (101 MHZ, DMSO) δ/ppm: 94.9 (C4 of pyrazole ring), 144.8 (C3 of pyrazole ring), 152.7 (C5 of pyrazole ring), 123.5 (CH=CH), 147.5 (CH=CH), 156.9 (C=Nimine), 157.7 (C=N pyridine ring), 123.4, 123.5, 124.4, 125.1, 125.5, 125.2, 126.3, 126.4, 126.85, 127.4, 127.6, 128.1, 128.5, 128.6, 129.2, 131.8, 132.6, 135.2, 136.4, 138.5, 139.3, 140.2 (Carom.); MS (EI) m/z: calculated for C37H25N5O2 [M]+, 571.62; found, M+-2 = 569.46, other fragments 522.66, 502.66 (base beak), 432.15, 384.41, 367.22, 296.15.
-
(3c): N-((5-(4-(dimethylamino)phenyl)-1-(pyridin-2-yl)penta-2,4-dien-1-ylidene)-3-(4-nitrophenyl) -1-phenyl-1H-pyrazol-5-amine
Reddish brown color; Yield: 3.73 g (69%); m.p = 130–133 °C: IR: ν/cm−1: 3100 (CH-Aro.)2919, (CH-Alip.), 1600 (C = N pyridine ring), 1517 (C=N imine), 1440 (C=Cchain); 1H NMR (400 MHz, d6- DMSO) δ/ppm: 7.56 (s, 1H, Pyrazole-H4), 6.61 (d, 1H, J = 6.54 Hz, CH=CH), 6.89 (d, 1H, J = 6.78 Hz, CH=CH), 6.67 (d, 1H, J = 6.52 Hz, CH=CH), 6.72 (d, 1H, J = 6.65 Hz, CH=CH), 7.59–8.79 (m, 4H, Py-H), 6.56- 8.38 (m, 13H, Ar–H). 13C NMR (101 MHZ, DMSO) δ/ppm: 41.9 (N-CH3), 94.9 (C4 of pyrazole ring), 144.7 (C3 of pyrazole ring), 152.5 (C5 of pyrazole ring), 131.1 (CH=CH), 146.6 (CH=CH), 157.9 (C=Nimine), 157.7 (C=N pyridine ring), 112.7, 123.4, 124.1, 125.2, 125.4, 126.8, 128.2, 129.5, 129.7, 131.8, 136.5, 138.7, 139.4, 147.6 149.1, 150.7 (Carom.).; MS (EI) m/z: calculated for C33H28N6O2 [M]+, 540.61; found, M+ + 1 = 541.67, other fragments 493.58, 446.49, 347.10, 280.52 (base beak), 172.74, 144.09.
Results and discussions
Catalyst characterization
FT-IR
The FT-IR spectrum of the Fe2O3 spindles was recorded in the range of 400–4000 cm−1. The large broad peak at 3453 cm−1 is ascribed to the O–H stretching vibration of OH groups. The absorption peaks around 1630, and 1382 cm−1 are due to the asymmetric and symmetric bending vibration of the C = O of the PVP stabilizing agent, indicating the formation of a PVP layer on the surface of spindles [25]. The band at 577 cm−1 is corresponding to the Fe–O stretching mode of Fe2O3, Fig. 5a [32]. For Fe2O3@SiO2, in addition to the previous band that appeared in the spectrum of Fe2O3 spindles, the new band around 1085 cm−1 is attributed to the symmetric and the asymmetric stretching vibration frequency of Si–O–Si, indicating the existence of SiO2 in the samples, Fig. 5b [27]. The intense peaks at 590, 553, and 440 cm−1 correspond to the In–O phonon vibration mode which is characteristic of In2O3 as shown in Fig. 5c [14, 33].
XRD
The crystallinity of Fe2O3, Fe2O3@SiO2, and Fe2O3@SiO2/In2O3 samples was determined by XRD analysis. Figure 6 shows the XRD pattern of Fe2O3 spindles. The peaks at 24°, 33o, 73°, and 75° can be attributed to (012), (104), (1010) and (220) planes of α-Fe2O3 [32, 34]. The peaks at 36o, 42o, 53o, 57o, and 62o can be attributed to the (311), (400), (422), (511), (440) of γ-Fe2O3 and therefore can be confirmed that the particles are a mixture of γ-Fe2O3 and α-Fe2O3 [35, 36]. After coating, hematite with SiO2, the peaks of the hematite remained unchanged but a new peak close to 2θ = 22.2o is developed. This confirms the presence of amorphous SiO2 [37, 38]. Figure 6 also displays the same characteristic peaks of hematite and SiO2, which are accompanied by many new peaks at 30°, 38°, 42°, 46°, 50°, 58°, 61°, and 78°. These peaks are assigned to (222), (411), (332), (431), (440), (541), (622), and (800) planes all of which indicate the deposition of In2O3 on the surface of Fe2O3@SiO2 core/shell [14, 39].
SEM
Figure 7a shows the morphology of the synthesized spindle-like particles. Figure 7b indicates that the SiO2 layer has successfully coated the surface of the hematite. Figure 7c shows a lot of hanging and brittle particles precipitated on the surface of the Fe2O3@SiO2 core/shell which indicates that In2O3 covered partially the surface of the core/shell and formed the Fe2O3@SiO2/In2O3 nanocomposite.
EDX
The energy-dispersive X-ray signals provide information on the sample composition. EDX spectra of the synthesized Fe2O3 spindles, Fe2O3@SiO2 core/shell, and Fe2O3@SiO2/In2O3 nanocomposite are shown in Fig. 8 where their tentative data are collected in Table 1. The data confirm the presence of Fe and O in Fe2O3 spindles, while Fe, O, Si in the Fe2O3@SiO2 core/shell and Fe, O, Si, In in Fe2O3@SiO2/In2O3 nanocomposite.
TEM
Figure 9a shows the morphology of the spindle-like particles of Fe2O3. The particles have an average length of 600 nm and width of 220 nm uniform and a continuous PVP layer appeared on the surface of the spindles, indicating that the obtained spindles are coated with the PVP. Figure 9b describes the TEM micrograph of the Fe2O3@SiO2 core/shell. It demonstrates that the Fe2O3 spindle core is covered with a SiO2 shell. In2O3 nanoparticles are present on the surface of the Fe2O3@SiO2 as hanging and brittle particles. Their average diameter is around 20 nm, Fig. 9c.
VSM
The magnetic hysteresis loops of the Fe2O3 spindles, Fe2O3@SiO2 core/shell, and Fe2O3@SiO2/In2O3 nanocomposite were measured by the VSM technique at room temperature and the results are shown in Fig. 10. The magnetic parameters of these samples were calculated from the individual M-H loops in Table 2. The VSM data of Fe2O3 spindles show saturation magnetic moment, Ms = 72.90 emu/g, at an applied magnetic field of 8000 G. It is accompanied by a small magnetic remanence (Mr = 2.81 emu/g) and very small coercively (Hc). Meanwhile, the values of Mr for the SiO2-coated spindles in Table 2 have not changed in comparison with the corresponding values of uncoated Fe2O3. Coating of Fe2O3 with the SiO2 reduces the value of Ms. Thus, the coated samples can easily be separated from the reaction mixture with an external magnet. Since the units of the magnetization are reported per gram of material, such a decrease in Ms would reflect a smaller percentage of net magnetic material per gram of the overall sample [40, 41].
Characterization of reaction products 3a–c
The azomethine compounds based on 5-amino-pyrazoles have been synthesized by two different methods, conventional and catalytic. The key start 3-(4-nitrophenyl)-1-phenyl-1H-pyrazole-5-amine (1) was synthesized in two steps: the first step is the reaction of sodium cyanide with 2-bromo-1-(4-nitrophenyl) ethanone to produce 3-(4-nitrophenyl)-3-oxopropanenitrile which is confirmed by the appearance of a nitrile absorption peak at 2255 cm−1. This product reacts in the second step with phenyl hydrazine in absolute ethanol and acetic acid as given in Fig. 11. The structure of compound 1 was identified by FT-IR, 1HNMR, 13CNMR, and mass spectrometry. The disappearance of the nitrile absorption peak in the FT-IR spectrum and the appearance of new broad bands at 3421 cm−1 and 3303 cm−1 due to the amino group supports the formation of this compound. The 1HNMR spectrum exhibits a characteristic methine proton (=CH of pyrazole ring) as a singlet at δ 5.58 ppm, an amino proton at δ 5.22 ppm which are exchangeable by mixing with D2O, and multiple at δ 7.22– 8.36 ppm that corresponding to aromatic protons. The 13CNMR spectrum of compound 1 has signals at δ 86.48, 146.79, and 148.46 ppm, indicating the presence of C3, C4, and C5 of the pyrazole ring, respectively. Furthermore, the formation of compound 1 has also been confirmed by mass spectra that match its molecular weight.
The starting compound 3-(4-nitrophenyl)-1-phenyl-1H-pyrazole-5-amine (1) was condensed with chalcones derivatives 2a–c by one-pot two-component systems in the presence of sulfuric acid to yield the corresponding compounds 3a–c (conventional method) or in the presence of Fe2O3@SiO2/In2O3 nanocatalyst (catalytic method) as shown in Fig. 12. The characteristic spectral analysis of FTIR, 1HNMR, 13CNMR, and mass spectra used to confirm the structures of the synthesized compounds are provided in the supplementary file (Figs. S1-S4).
The first reversible step of the mechanism of azomethine formation is the nucleophilic attack of the amine group on the electrophilic carbonyl carbon of the aldehyde to form an imine. The formation of azomethine from an imine depends largely on the water removal rate in the final step.
Depictions of compounds 3a–c were established based on their elemental analyses and spectral data (FTIR, 1H, 13CNMR, and MS). The FT-IR absorption bands of the compounds 3a–c appeared at 1600–1673 cm−1 for the C=N pyridine ring, and 1510-1517 cm−1 are due to azomethine group C=N– and the 1440–1510 cm−1 are due to the CH=CHaliphatic for compounds 3a–c, respectively. The 1H NMR spectra revealed doublet signals at range δ 7.57–7.62 ppm which are due to the CH=CH. The doublet signals at range δ 6.72–7.75 ppm are attributed to the CH=CH for the compounds 3a–c. For the compound 3c, the singlet signal at 2.87 ppm is due to N-CH3, the doublet signals at δ 6.61 ppm are characteristic of the CH=CH, and the doublet signals at δ 6.89 ppm are assignable to the CH=CH. The signal of the free NH2 protons is absent in the spectra of azomethine compounds 3a–c indicating their formation. All other protons are located at their respective positions. 13C NMR spectra of compounds 3a–c showed signals at δ 123.5–131.1 that belong to the (CH=CH), signals at δ 139.3–147.5 correspond to the (CH=CH), the signals at δ 156.2–157.9 are due to (C=Nimine), the signals at δ 157.7 are attributed to the (C=N of pyridine ring). The number of signals found are corresponding to the magnetically nonequivalent carbon atoms. Moreover, the mass spectra of the azomethine compounds 3a–c displayed peaks at m/z which match their exact molecular mass. Details of selected spectroscopic data are reported in the experimental section.
Comparative synthesis of compounds 3(a-c), comparative study
Many studies on the synthesis of azomethine compounds have been reported [42, 43], but they all suffer from one or more serious shortcomings, such as high environmental pollution due to the solvent and reaction hardness, high temperature, multi-step pathways, and long reaction times with low-to-moderate yields under severe reaction conditions. Recently, one of the most intriguing areas in the synthesis of widely used organic compounds is the focus on benign environmentally friendly conditions and reagents such as catalysts. The catalysts have played vital roles in reducing the pollution of our environment. In addition, the catalyst can be recycled from the liquid reaction medium and reused, to maintain the high productivity of reaction products [44, 45].
In this regard, we studied the condensation reaction between 3-(4-nitrophenyl)-1-phenyl-1H-pyrazole-5-amine (1) and chalcones derivatives 2a–c by one-pot two-component systems using the heterogeneous nanocatalyst, Fe2O3@SiO2/In2O3 to synthesize a series of 3-(substituted)-1-(pyridine-2-yl) allylidene)-3-(4-nitrophenyl)-1-phenyl-1H-pyrazole-5-amine derivatives 3a–c. The desired products were obtained with high yields in short reaction times. The suggested mechanism offers the removal of water by the dehydration strategy [46]. The Fe2O3@SiO2/In2O3 catalyst was used as a dehydrating agent for the conversion of the imine into the corresponding azomethine products. The reaction is facile due to the good electrophilic and nucleophilic properties of the carbonyl and amine groups, respectively. The comparative results are depicted in Table 3. The results showed that the synthesized compounds by different techniques have the same properties [TLC, melting point, and mixed melting point] with more cleanly and high yield in the case of the catalytic method than the conventional one.
The comparison of the results obtained from the Fe2O3@SiO2/In2O3 catalyzed method with some of the reported catalysts used for the synthesis of Schiff bases shows the high efficiency of the Fe2O3@SiO2/In2O3 catalyzed method. This is due to the good yield and the short reaction time as given in Table 4.
Recycling of nanocatalyst
To examine the reusability of Fe2O3@SiO2/In2O3 nanocatalyst, it was magnetically recovered from the reaction mixture and reused for fresh subsequent experiments under the same reaction conditions. It is interesting to know that the yields remained approximately constant during all the experimental runs as illustrated in Fig. 13. The recyclability and reusability of the catalyst have been established without a significant loss of its activity. The stability of the catalyst after the first three cycles has been examined by the analysis of the reused catalyst with FTIR, XRD, SEM, and TEM, Fig. (S5). The results showed no change from the fresh catalyst, indicating its high stability.
Conclusion
The application of Fe2O3@ SiO2/In2O3 as a nanocatalyst in the preparation of some Schiff bases that have many applications in biological fields was a successful approach. The addition of the nanocatalyst into the reaction medium during the preparation of these materials led to a significant increase in the formation rate of these compounds over that of noncatalyzed reactions that proceeded under only the effect of heat. It is worth noting that the completion time of noncatalyzed reactions decreased from 5–8 h to 20–30 min upon the incorporation of the nanocatalyst. The formation percentages of final products have also increased from 69–77% (conventional) to 90–92% (catalyzed). A further advantage associated with the Fe2O3@SiO2/In2O3 when used in the present organic synthesis is not only the formation of the same final products yielded from the conventional routes, but also acquiring high purity for such products. These interesting properties qualify this nanocatalyst for wider applications in the field of organic synthesis. In conclusion, we introduce an efficient method for the synthesis of Schiff bases with easy workup, facile conditions, fast reaction rates, good yields, and high selectivity. This novel nanocatalyst is promising for the development of organic synthesis in a facile way.
Availability of data and materials
No data supporting the findings of this study are available in the supporting information of this article.
References
N. Rahman and R. Nongkhlaw, Org. Chem. 272 (2018).
C. Burda, X. Chen, R. Narayanan, M.A. El-Sayed, Chem. Rev. 105, 1025 (2005)
S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. Vander Elst, R.N. Muller, Chem. Rev. 110, 2573 (2010)
Q.L. Zhu, Q. Xu, Chem 1, 220 (2016)
P. Yang, L. Yang, Q. Gao, Q. Luo, X. Zhao, X. Mai, Q. Fu, M. Dong, J. Wang, Y. Hao, R. Yang, X. Lai, S. Wu, Q. Shao, T. Ding, J. Lin, Z. Guo, Chem. Commun. 55, 9011 (2019)
Y.A. Titova, O.V. Fedorova, G.L. Rusinov, V.N. Charushin, Russ. Chem. Rev. 84, 1294 (2015)
R. Zboril, M. Mashlan, D. Petridis, Chem. Mater. 14, 969 (2002)
N. Basavegowda, K. Mishra, Y.R. Lee, Adv. Nat. Sci. Nanosci. Nanotechnol. 8, 25017 (2017)
B. Maleki, H. Alinezhad, H. Atharifar, R. Tayebee, A.V. Mofrad, Org. Prep. Proced. Int. 51, 301 (2019)
S.A.M. Ziabari, M. Babamoradi, Z. Hajizadeh, A. Maleki, Eurasian Chem. Commun. 2, 456 (2020)
L.T. Gibson, Chem. Soc. Rev. 43, 5173 (2014)
S. Kachbouri, E. Elaloui, Y. Moussaoui, Iran. J. Chem. Chem. Eng. 38, 17 (2019)
F. Laffafchi, M. Tajbakhsh, Y. Sarrafi, M. Ghani, B. Maleki, J. Sep. Sci. 45, 3005 (2022)
J. Chandradass, D. Sik, K. Hyeon, Adv. Powder Technol. 22, 370 (2011)
U. Mandal, S. Mandal, B. Chakraborty, C. Rizzoli, D. Bandyopadhyay, Polyhedron 177, 114320 (2020)
A. Rambabu, M. Pradeep Kumar, N. Ganji, S. Daravath, Shivaraj, J. Biomol. Struct. Dyn. 38, 307 (2020)
K. Buldurun, N. Turan, E. Bursal, A. Mantarcı, F. Turkan, P. Taslimi, İ Gülçin, Res. Chem. Intermed. 46, 283 (2020)
M.M. Miloud, M.M. El-ajaily, T.H. Al-noor, N.S. Al-barki, J Bacteriol Mycol 7, 1122 (2020)
Y. Liu, W. Deng, Z. Meng, W. Wong, Small 16, 1905204 (2020)
P.S. Chandrachood, A.R. Jadhav, D.R. Garud, N.R. Deshpande, V.G. Puranik, R.V. Kashalkar, Res. Chem. Intermed. 46, 5219 (2020)
Z. Jalili, R. Tayebee, F.M. Zonoz, RSC Adv. 11, 18026 (2021)
B. Maleki, H. Natheghi, R. Tayebee, H. Alinezhad, A. Amiri, S.A. Hossieni, S.M.M. Nouri, Polycycl. Aromat. Compd. 40, 633 (2020)
H.R. Saadati-Moshtaghin, B. Maleki, R. Tayebee, S. Kahrobaei, F. Abbasinohoji, Polycycl. Aromat. Compd. 42, 885 (2022)
S. Sajjadifar, I. Amini, S. Habibzadeh, G. Mansouri, E. Ebadi, Chem. Methodol. 4, 624 (2020)
Y. Zhang, L. Ma, T. Wang, X. Li, Fuel 177, 197 (2016)
L. Sun, W. Wu, S. Yang, J. Zhou, M. Hong, X. Xiao, F. Ren, C. Jiang, ACS Appl. Mater. Interfaces 6, 1113 (2014)
G. Lin, H. Wang, X. Li, X. Lai, Y. Zou, X. Zhou, D. Liu, J. Wan, H. Xin, Sensors Actuators B Chem. 255, 3364 (2018)
T.A. Fayed, M.K. Awad, Chem. Phys. 303, 317 (2004)
M.N. El-Nahass, D.M. Abd El-Aziz, T.A. Fayed, Sensors Actuators B Chem. 205, 377 (2014)
J.S. Jadhav, K.R. Bodawar, G.V. Panchal, Int. J. TechnoChem Res. 1, 97 (2015)
M. Arifuzzaman, M.R. Karim, T.A. Siddiquee, A.H. Mirza, M.A. Ali, Int. J. Org. Chem. 3, 81 (2013)
M. Farahmandjou, F. Soflaee, Phys. Chem. Res. 3, 191 (2015)
M. Jothibas, C. Manoharan, S. Johnson Jeyakumar, P. Praveen, J. Mater. Sci. Mater. Electron. 26, 9600 (2015)
D. Garcia, G. Picasso, P. Hidalgo, H.E.M. Peres, R. Sun Kou, J.M. Gonçalves, Anal. Chem. Res. 12, 74 (2017)
D. Cao, H. Li, L. Pan, J. Li, X. Wang, P. Jing, Sci. Rep. 6, 32360 (2016)
W. Wu, X.H. Xiao, S.F. Zhang, T.C. Peng, J. Zhou, F. Ren, C.Z. Jiang, Nanoscale Res. Lett. 5, 1474 (2010)
D.B. Tada, L.L.R. Vono, E.L. Duarte, R. Itri, P.K. Kiyohara, M.S. Baptista, L.M. Rossi, Langmuir 23, 8194 (2007)
M. Zhang, B. L. Cushing, and C. J. O’Connor, Nanotechnology 19, (2008).
D. N. Chavan, G. E. Patil, D. D. Kajale, V. B. Gaikwad, P. K. Khanna, and G. H. Jain, J. Sensors 2011, (2011).
C.R. Vestal, Z.J. Zhang, J. Am. Chem. Soc. 124, 14312 (2002)
H.G. El-Attar, M.A. Salem, E.A. Bakr, Appl. Organomet. Chem. 35, e6357 (2021)
T.Y. Fonkui, M.I. Ikhile, P.B. Njobeh, D.T. Ndinteh, BMC Chem. 13, 1 (2019)
S.G. Nayak, B. Poojary, Heliyon 5, e02318 (2019)
D. Fiorito, S. Scaringi, and C. Mazet, Chem. Soc. Rev. (2021).
M. Mandal, A.N. Chattopadhyay, A.P. Chattopadhyay, M.O.J. Bioorg, Org. Chem. 2, 201 (2018)
M.A. Bedair, S.A. Soliman, M.F. Bakr, E.S. Gad, H. Lgaz, I.-M. Chung, M. Salama, F.Z. Alqahtany, J. Mol. Liq. 317, 114015 (2020)
L. Ravishankar, S.A. Patwe, N. Gosarani, A. Roy, Synth. Commun. 40, 3177 (2010)
H. Naeimi, F. Salimi, K. Rabiei, J. Mol. Catal. A Chem. 260, 100 (2006)
A.K. Chakraborti, S. Bhagat, S. Rudrawar, Tetrahedron Lett. 45, 7641 (2004)
G.C. Look, M.M. Murphy, D.A. Campbell, M.A. Gallop, Tetrahedron Lett. 36, 2937 (1995)
F. Texier-Boullet, Synth. 679 (1985).
R.S. Varma, R. Dahiya, S. Kumar, Tetrahedron Lett. 38, 2039 (1997)
S.S. Kumbar, K.M. Hosamani, G.C. Gouripur, S.D. Joshi, R. Soc, Open Sci. 5, 172416 (2018)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work received no funding from any source.
Author information
Authors and Affiliations
Contributions
H.G. El-Attar involved in conceptualization, methodology, formal analysis, investigation, data curation, writing, and editing of the original draft. M.A. Salem involved in conceptualization, supervision, formal analysis, investigation, and writing—review and editing. S.A. Ibrahim involved in methodology, formal analysis, and writing the original draft. E.A. Bakr involved in conceptualization, formal analysis, investigation, data curation, writing, and editing of the original draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declared that there is no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Attar, H.G., Salem, M.A., Ibrahim, S.A. et al. Highly efficient and recyclable novel spindles Fe2O3@SiO2/In2O3 nanomagnetic catalyst designed for green synthesis of azomethine compounds. Res Chem Intermed 49, 469–489 (2023). https://doi.org/10.1007/s11164-022-04894-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04894-4