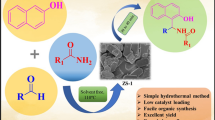
Abstract
Zeolite ZSM-11 catalyst was prepared by hydrothermal method and characterized by FTIR, XRD, SEM, HRTEM, EDS, and BET analysis techniques. The catalyst shows good catalytic activity toward synthesis of 2,4,5-triarylimidazole derivatives which is prepared by using benzil, aldehyde and ammonium acetate in solvent-free condition. The reaction, one pot synthesis is highly adaptable and eco-friendly and has several merits such as short reaction time, mild reaction conditions, and high yield. The ease of reusability and recovery of catalyst for five consecutive reactions makes this protocol highly suitable.
Graphical abstract










Similar content being viewed by others
References
S. Dehghan Khalili, S.H. Banitaba, J. Safari, Sci. Iran. 20, 1855 (2013)
M. Esmaeilpour, J. Javidi, M. Zandi, New J. Chem. 39, 3388 (2015)
H. Naeimi, D. Aghaseyedkarimi, New J. Chem. 39, 9415 (2015)
J. Jayram, V. Jeena, Green Chem. 19, 5841 (2017)
A. Shaabani, R. Afshari, S.E. Hooshmand, New J. Chem. 41, 8469 (2017)
Y. Chen, R. Wang, F. Ba, J. Hou, A. Ding, M. Zhou, H. Guo, J. Saudi Chem. Soc. 21, 76 (2017)
A. Shaabani, A. Maleki, M. Behnam, Synth. Commun. 39, 102 (2009)
J. Jayram, V. Jeena, RSC Adv. 8, 37557 (2018)
S.A. Siddiqui, U.C. Narkhede, S.S. Palimkar, T. Daniel, R.J. Lahoti, K.V. Srinivasan, Tetrahedron 61, 3539 (2005)
J. Wang, R. Mason, D. VanDerveer, K. Feng, X.R. Bu, J. Org. Chem. 68, 5415 (2003)
S. Sarshar, D. Siev, A.M.M. Mjalli, Tetrahedron Lett. 37, 835 (1996)
X.C. Wang, H.P. Gong, Z.J. Quan, L. Li, H.L. Ye, Chinese Chem. Lett. 20, 44 (2009)
S.N. Murthy, B. Madhav, Y.V.D. Nageswar, Tetrahedron Lett. 51, 5252 (2010)
A. Shaabani, A. Rahmati, J. Mol. Catal. A Chem. 249, 246 (2006)
M.G. Shen, C. Cai, W. Bin Yi, J. Fluor. Chem. 129, 541 (2008)
L.M. Wang, Y.H. Wang, H. Tian, Y.F. Yao, J.H. Shao, B. Liu, J. Fluor. Chem. 127, 1570 (2006)
H. Weinmann, M. Harre, K. Koenig, E. Merten, U. Tilstam, Tetrahedron Lett. 43, 593 (2002)
N.D. Kokare, J.N. Sangshetti, D.B. Shinde, Synthesis (Stuttg). (2007). https://doi.org/10.1055/s-2007-983872
M.M. Khodaei, K. Bahrami, I. Kavianinia, J. Chinese Chem. Soc. 54, 829 (2007)
T.L.M. Maesen, M. Schenk, T.J.H. Vlugt, B. Smit, J. Catal. 203, 281 (2001)
Y. Gu, N. Cui, Q. Yu, C. Li, Q. Cui, Appl. Catal. A Gen. 429–430, 9 (2012)
Q. Yu, C. Cui, Q. Zhang, J. Chen, Y. Li, J. Sun, C. Li, Q. Cui, C. Yang, H. Shan, J. Energy Chem. 22, 761 (2013)
L. Zhang, H. Liu, X. Li, S. Xie, Y. Wang, W. Xin, S. Liu, L. Xu, Fuel Process. Technol. 91, 449 (2010)
P.M. Piccione, M.E. Davis, Microporous Mesoporous Mater. 49, 163 (2001)
G.T. Kokotailo, P. Chu, S.L. Lawton, W.M. Meier, Comptes Rendus Chimie 275, 119 (1978)
X. Wang, F. Meng, H. Chen, F. Gao, Y. Wang, X. Han, C. Fan, C. Sun, S. Wang, L. Wang, Comptes Rendus Chim. 20, 1083 (2017)
K.P. Dey, S. Ghosh, M.K. Naskar, Ceram. Int. 39, 2153 (2013)
G. Coudurier, C. Naccache, J.C. Vedrine, J. Chem. Soc. Chem. Commun. (1982). https://doi.org/10.1039/c39820001413
W. Song, Z. Liu, L. Liu, A.L. Skov, N. Song, G. Xiong, K. Zhu, X. Zhou, RSC Adv. 5, 31195 (2015)
M.M.J. Treacy, J.B. Higgins (eds.), Collection of Simulated XRD Powder Patterns for Zeolites, 5th edn. (Amsterdam, Elsevier, 2007), p. 477. https://doi.org/10.1016/B978-0-444-53067-7.X5470-7
K. Shen, N. Wang, X. Chen, Z. Chen, Y. Li, J. Chen, W. Qian, F. Wei, Catal. Sci. Technol. 7, 5143 (2017)
J. Yang, S. Yu, H. Hu, Y. Zhang, J. Lu, J. Wang, D. Yin, Chem. Eng. J. 166, 1083 (2011)
S. S. Lapari, Z. Ramli, S. Triwahyono, 2015 (2015).
H. Chen, X. Zhang, J. Zhang, Q. Wang, RSC Adv. 7, 46109 (2017)
M. Kidwai, P. Mothsra, V. Bansal, R. Goyal, Monatshefte fur Chemie 137, 1189 (2006)
S. Balalaie, A. Arabanian, M.S. Hashtroudi, Monatshefte fur Chemie 131, 945 (2000)
H.D. Hanoon, S.M. Radhi, S.K. Abbas, A.I.P. Conf, Proc. 2144, 1 (2019)
V.S.V. Satyanarayana, A. Sivakumar, Chem. Pap. 65, 519 (2011)
A.A. Marzouk, V.M. Abbasov, A.H. Talybov, S.K. Mohamed, World. J. Org. Chem. 1, 6 (2013)
J. Safari, Z. Zarnegar, Ultrason. Sonochem. 20, 740 (2013)
G.V.M. Sharma, Y. Jyothi, P.S. Lakshmi, Synth. Commun. 36, 2991 (2006)
A. Teimouri, A.N. Chermahini, J. Mol. Catal. A Chem. 346, 39 (2011)
H.D. Hanoon, E. Kowsari, M. Abdouss, M.H. Ghasemi, H. Zandi, Res. Chem. Intermed. 43, 4023 (2017)
L. Wang, C. Cai, Monatshefte fur Chemie 140, 541 (2009)
B.F. Mirjalili, A. Bamoniri, M.A. Mirhoseini, Sci. Iran. 20, 587 (2013)
Acknowledgements
One of the authors, Mr. Sudarshan S. Dipake, gratefully thankful to the Council of Scientific and Industrial Research (CSIR), New Delhi for the award of fellowship and to the Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad-431004 (M.S.), India. for support and providing the necessary laboratory facility.
Author information
Authors and Affiliations
Contributions
SSD-Conduct the whole experiment, Writing original draft. MKL-Review and editing. ASR-Review and editing. STG-Principal author.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Consent to participate
All Authors are agreed for submission.
Consent for publication
Agreed to submission.
Data availability
Manuscript including all data correct and unpublished.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dipake, S.S., Lande, M.K., Rajbhoj, A.S. et al. Zeolite ZSM-11 as a reusable and efficient catalyst promoted improved protocol for synthesis of 2,4,5-triarylimidazole derivatives under solvent-free condition. Res Chem Intermed 47, 2245–2261 (2021). https://doi.org/10.1007/s11164-021-04423-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04423-9