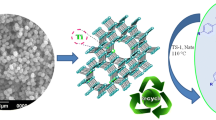
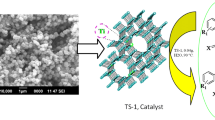
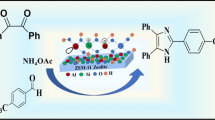
Abstract
A new method is described for one-pot solvent-free synthesis of 2,4,6-triaryl pyridines in the presence of a solid acid catalyst, titanium silicate (TS-1) via cyclocondensation of acetophenone, aryl aldehyde and ammonium acetate. The present method illustrates several advantages, such as eco-friendly reaction conditions, simplicity, short reaction time (3 h), easy separation of catalyst and high yields of the products (85–93%). Furthermore, the TS-1 catalyst was reused for four catalytic cycles with consistent catalytic activity.
Graphical Abstract





Similar content being viewed by others
References
W. Zhao, F.E. Chen, Cur. Org. Syn. 9, 873 (2012)
P. Martins, J. Jesus, S. Santos, L.R. Raposo, C.R. Rodrigues, P.V. Baotusta, A.R. Fernandes, Molecules 20, 16852 (2015)
A. Ahmad, A. Husain, S.A. Khan, M. Mujeeb, A. Bhandari, J. Saudi Chem. Soc. 20, 577 (2016)
R. Karki, C. Park, K.Y. Jun, J.G. Jee, J.H. Lee, P. Thapa, T.M. Kadayat, Y. Kwon, E.S. Lee, Eur. J. Med. Chem. 84, 555 (2014)
S. Samshuddin, B. Narayana, D.N. Shettyand, R. Raghavendra, Der Pharma Chemika 3, 232 (2011)
R. Khajuria, P. Kannaboina, K.K. Kapoor, A. Gupta, G. Raina, A.K. Jassal, L.K. Rana, M.S. Hundal, P. Das, Org. Biomol. Chem. 13, 5944 (2015)
G. Bist, S. Park, C. Song, T.B.T. Magar, A. Shrestha, Y. Kwon, E.S. Lee, Eur. J. Med. Chem. 133, 69 (2017)
A.R. Moosavi-Zare, M.A. Zolfigol, S. Farahmand, A. Zare, A.R. Pourali, R. Ayazi-Nasrabadi, Synlett 25, 193 (2014)
L. Yasmin, P.K. Eggers, B.W. Skelton, K.A. Stubbs, C.L. Raston, Green Chem. 16, 3450 (2014)
V. Kimesova, M. Sovoboda, K. Waisser, M. Pour, J. Kaustova, IL Farmaco 54, 666 (1999)
I.J. Enyedy, S. Sakamuri, W.A. Zaman, K.M. Johnson, S. Wang, Med. Chem. Lett. 13, 513 (2003)
A. Winter, C. Friebe, M.D. Hager, U. S. Schubert, Ero. J. Org. Chem. (6), 801 (2009)
M.M. Heravi, K. Bakhtiari, Z. Daroogheha, F.F. Bamoharram, Catal. Commun. 8, 1991 (2007)
H. Alinezhad, M. Tajbakhsh, N. Ghobadi, Syn. Commun. 45, 1964 (2015)
M.R.M. Shafiee, R. Moloudi, J. Chem. Res. 35, 294 (2011)
P.V. Shende, V.B. Labade, J.B. Gujar, B.B. Shingte, M.S. Shingare, Tetrahedron Lett. 53, 1523 (2012)
M.R.M. Shafiee, R. Moloudi, M. Ghashang, APCBEE Procedia 1, 221 (2012)
P. Rajput, N.J.P. Subhashini, Shivaraj, J. Sci. Res. 2, 337 (2010)
E. Tabrizian, A. Amoozadeh, S. Rahmani, E. Imanifar, S. Azhari, M. Malmir, Chi. Chem. Lett. 26, 1278 (2015)
M.R.M. Shafiee, R. Moloudi, Lett. Org. Chem. 8, 719 (2011)
R.S. Rohokale, B. Koenig, D.D. Dhavale, J. Org. Chem. 81, 7121 (2016)
M. Kamli, Cogent Chem. 2, 1171123 (2016)
V. Kannan, K. Sreekumar, Mod. Res. Catal. 2, 42 (2013)
J. Safari, S.G. Ravandi, M.B. Borujeni, J. Chem. Sci. 125, 1063 (2013)
Z. Zarnegar, J. Safari, M.B. Borujeni, Chem. Hete. Comp. 501, 683 (2015)
Z.L. Min, T.Z. Yin, X.M. Hu, Asian J. Chem. 26, 7977 (2014)
S. Mahernia, M. Adib, M. Mahdavi, M. Nosrati, Tetrahedron Lett. 55, 3844 (2014)
A. Thangaraj, M.J. Eapen, S. Sivasanker, P. Ratnasamy, Zeolites 12, 943 (1992)
M. Taramasso, U. S. Patent 4, 410 (1983)
H.F. Youssef, W.H. Hegazy, H.H. Abo-almaged, G.T. El-Bassyouni, Bioinorg. Chem. App. 12, 428121 (2015)
M. Opanasenko, A. Dhakshinamoorthy, M. Shamzhy, P. Nachtigall, M. Horacek, H. Garcia, J. Cejka, J. Catal. Sci. Technol. 3, 500 (2013)
N. Wilde, M. Pelz, S.G. Gebhardt, R. Glaser, Green Chem. 17, 3378 (2015)
M.G. Clerici, O.A. Kholdeeva, Liquid Phase Oxidation Via Heterogeneous Catalysis, Firsted (Wiley, Hoboken, 2013)
Y. Hayashi, Chem. Sci 7, 866 (2016)
C. Abraham, G.M. Dmitry, K. Steven, B. Timothy, D.B. Jonathan Milton, J. Solvent Extr. Ion Exch. 30, 229 (2012)
K. Moller, T. Bein, Chem. Soc. Rev. 42, 3689 (2013)
C.K. Banerjee, J.D. Umarye, P.R. Kanjilal, Synth. Commun. 43, 2208 (2013)
E.G. Derouane, J.C. Vedrine, R.R. Pinto, M.P. Borges, L. Costa, M.A.N.D.A. Lemos, F. Lemos, F.R. Ribeiro, Catal. Rev. Sci. Eng. 55, 454 (2013)
J. Zhuaung, Z. Yan, X. Liu, X. Liu, X. Han, X. Bao, U. Mueller, Catal. Lett. 83, 1 (2002)
S. P. Gadekar, G. T. Pawar, R. R. Magar, M. K. Lande, Polycycl. Aromat. Compd. (2017)
G.T. Pawar, S.P. Gadekar, B.R. Arbad, M.K. Lande, Bull. Chem. Rea. Eng. Catal. 12, 32 (2016)
M.M. Treacy, J.B. Higgins, Collection of Simulated XRD Powder Patterns for Zeolites (Elsevier, Amsterdam, 2001), p. 236
Y. Xue, Y. Xie, H. Wei, Y. Wen, X. Wang, B. Li, New J. Chem. 38, 4229 (2014)
X. Wang, Y. Guo, X. Zhang, Y. Wang, H. Liu, J. Wang, J. Qiua, K.L. Yeung, Chem. Eng. J. 156, 562 (2010)
R. Barakov, N. Shcherban, P. Yaremov, V. Solomakha, A. Yshnevskyy, V. Ilyin, J. Porous Mat. 23, 517 (2016)
A.B. Gambhire, M.K. Lande, S.B. Rathod, B.R. Arbad, K.N. Vidhate, R.S. Gholap, K.R. Patil, Arab. J. Chem. 6, s429 (2016)
G. Ricchiardi, A. Damin, S. Bordiga, C. Lamberti, G. Spano, F. Rivetti, A. Zecchinna, J. Am. Chem. Soc. 123, 11409 (2001)
A.C.K. Yip, F.L.Y. Lam, X. Hu, P. Li, W.K. Yuan, Ind. Eng. Chem. Res. 48, 5266 (2009)
Acknowledgements
We gratefully acknowledge the Head, Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad–431004 (MS), India, for his support and constant encouragement during the progress of this work and for providing necessarily laboratory facilities. The corresponding author, MKL, is grateful to UGC New Delhi for providing financial support under major research project F. No. 43-184/2014 (SR). The authors are grateful to STIC Cochin and SAIF Chandigarh for characterization facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11164_2018_3305_MOESM1_ESM.docx
Full experimental details and 1H, 13C NMR, HRMS spectra, XRD. BET, SEM FTIR, of other products in the “supplementary content” of this article web page are available. (DOCX 1110 kb)
Rights and permissions
About this article
Cite this article
Gadekar, S.P., Lande, M.K. Solid acid catalyst TS-1 zeolite-assisted solvent-free one-pot synthesis of poly-substituted 2,4,6-triaryl-pyridines. Res Chem Intermed 44, 3267–3278 (2018). https://doi.org/10.1007/s11164-018-3305-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3305-4