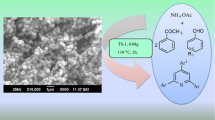
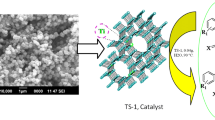
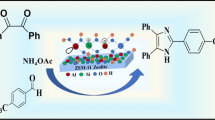
Abstract
Titanium silicate (TS-1) zeolite heterogeneous catalyst is synthesized by the hydrothermally discontinuous method and is characterized by using XRD, SEM, TEM, and NH3–TPD techniques. The catalytic activity of the TS-1 type zeolite was tested for one-pot solvent-free synthesis of 1,3-thiazolidine-4-ones. The present technique illustrates many benefits, including eco-friendly reaction conditions, environmentally helpful, short response time, simplicity, straightforward separatation, catalyst reusability and high yields of the products. Furthermore, the catalyst was utilized for four recycle reactions and it has been found that the catalyst shows consistent chemical process activity.
Graphical abstract







Similar content being viewed by others
References
A. Pragi, A. Varun, H.S. Lamba, D. Wadhwa, IJPSR 3(09), 2947 (2012)
H. Bhatt, S. Sharma, Arab. J. Chem. 10, S531 (2017)
N. Siddiqui, M.F. Arshad, S.A. Khan, W.J. Ahsan, Enzyme Inhib. Med. Chem. 25(4), 485 (2010)
A.K. Jain, A. Vaidya, V. Ravichandran, S.K. Kashaw, R.K. Agrawal, Bioorg. Med. Chem. 20(11), 3378 (2012)
A. Verma, S.K. Saraf, Eur. J. Med. Chem. 43(5), 897 (2008)
V. Opletalova, J. Dolezel, J. Kunes, V. Buchta, M. Vejsova, M. Kucerova-Chlupacova, Molecules 21, 1592 (2016)
C.M. Bhalgat, P.V. Darda, K.G. Bothara, S.V. Bhandari, J. Gandhi, B. Ramesh, Euro. J. Med. Chem. 76, 580 (2014)
A. Gupta, R. Singh, P.K. Sonar, S.K. Saraf, Biochem. Res. Int. 2016, 8086762 (2016)
D.D. Subhedar, M.H. Shaikh, M.A. Arkile, A. Yeware, D. Sarkar, B.B. Shingate, Bioorg. Med. Chem. 26, 1704 (2016)
G. Cihan-Ostundag, E. Gursoy, L. Naesens, N. Ulusoy-Guzeldemirci, G. Capan, Bioorg. Med. Chem. 1524(2), 240 (2016)
K. Appalanaidu, R. Kotcherlakota, T.L. Dadmal, V.S. Bollu, R.M. Kumbhare, C.R. Patra, Bioorg. Med. Chem. 26, 5361 (2016)
C.D. Dago, C.N. Ambeu, W.K. Coulibaly, Y.A. Békro, J.A. Mamyrbekova-Bekro, R.L. Guével, A. Corlu, J.P. Bazureau, Chem. Heterocycl. Compd. 53(3), 341 (2017)
D.N. Pansare, N.A. Mulla, C.D. Pawar, V.R. Shende, D.B. Shinde, Bioorg. Med. Chem. 24, 3569 (2014)
A.T. Panzariu, M. Apotrosoaei, I.M. Vasincu, M. Drăgan, S. Constantin, F. Buron, S. Routier, L. Profire, C. Tuchilus, Chem. Cent. J. 10, 6 (2016)
S.J. Gilani, S.A. Khan, O. Alam, V. Singh, A. Arora, J. Serb. Chem. Soc. 76(8), 1057 (2011)
A. Bielenica, G. Sanna, S. Madeddu, M. Struga, M. Jozwiak, M. Koziol, A.E.G. Sawczenko, A.G. Materek, A. Serra, G. Giliberti, Chem. Biodrug. Des. 90(5), 883 (2017)
A. Bielenica, G. Sanna, S. Madeddu, M. Struga, M. Jóźwiak, A.E. Kozioł, A. Sawczenko, I.B. Materek, A. Serra, G. Giliberti, Chem. Biol. Drug Des. 92(1), 1157 (2017)
I.M. Vasincu, M. Apotrosoaei, A.T. Panzariu, F. Buron, S. Routier, L. Profire, Molecules 19, 15005 (2014)
K. Liaras, M. Fesatidou, A. Geronikaki, Molecules 23, 685 (2018)
S.J. Gilani, K. Nagarajan, S.P. Dixit, M. Taleuzzaman, S.A. Khan, Arab. J. Chem. 9, S1523 (2016)
P. Samadhiya, R. Sharma, S.K. Srivastava, S.D. Srivastava, Arab. J. Chem. 7, 657 (2014)
F.T. Tugcu, K. Turhan, M. Karadayi, M. Gulluce, Rom. Biotechnol. Lett. 10, 1 (2017)
Y. Hayashi, Chem. Sci. 7, 866 (2016)
P. Martins, J. Jesus, S. Santos, L.R. Raposo, C.R. Rodrigues, P.V. Baptista, A.R. Fernandes, Molecules 20, 16852 (2015)
J.S. Ghomi, M. Navvab, H.S. Alavi, Ultrason. Sonochem. 31, 102 (2015)
M. Apotrosoaei, I.M. Vasincu, M. Dragan, F. Buron, S. Routier, L. Profire, Molecules 19, 13824 (2014)
D. Gautam, P. Gautam, R.P. Chaudhary, Chin. Chem. Lett. 23, 1221 (2012)
S. Ebrahimi, J. Sulfur Chem. 37(6), 587 (2016)
M. Mamaghani, M. Pourranjbar, R.H. Nia, J. Sulfur Chem. 35(1), 1 (2014)
N. Foroughifar, S. Ebrahimi, Chin. Chem. 24, 389 (2013)
N. Sadou, S.A. Bouzroura, R. Nechak, B.N. Kolli, V. Morizur, S.P. Martini, E. Dunach, Polycycl. Aromat. Compd. 36, 1–11 (2016)
J.S. Ghomi, M. Navvab, H.S. Alavi, J. Sulfur Chem. 37(6), 601 (2016)
J. Luo, Z. Zhong, H. Ji, J. Chen, J. Zhao, F.J. Zhang, Sulfur Chem. 37(4), 438 (2016)
M. Zhengyue, Mod. Appl. Sci. 5, 3 (2011)
Z. Zhuo, L. Wu, L. Wang, Y. Ding, X. Zhang, Y. Liu, M. He, RSC Adv. 4, 55685 (2014)
J. Zhuang, Z. Yan, X. Liu, X. Liu, X. Han, X. Bao, U. Mueller, Cat. Lett. 83, 1 (2002)
T. Bucko, J. Hafner, L. Benco, J. Chem. Phys. 120(21), 10263 (2004)
D. Serrano, R. Sanz, P. Pizarro, I. Moreno, Chem. Commun. 11, 1407 (2009)
X. Deng, Y. Wang, L. Shen, H. Wu, Y. Liu, M. He, Ind. Eng. Chem. Res. 52, 1190 (2013)
Y. Luo, J. Xiong, C. Pang, G. Li, C. Hu, Catalysts 8, 49 (2018)
S. Gadekar, G. Pawar, R. Magar, M. Lande, Polycycl. Arom. Compd. 1 (2017)
M.M. Treacy, J.B. Higgins, Collection of Simulated XRD Powder Patterns for Zeolites, 4th edn. (Elsevier, Amsterdam, 2001)
Y. Xue, Y. Xie, H. Wei, Y. Wen, X. Wang, B. Li, New J. Chem. 38, 4229 (2014)
S.M. Sadeghzadeh, M. Malekzadeh, J. Mol. Liq. 202, 46–51 (2015)
A. Bolognese, G. Correale, M. Manfra, A. Lavecchia, E. Novellino, V. Barone, Org. Biomol. Chem. 2, 2809–2813 (2004)
J. Harindran, IJRPC 4(2), 351 (2014)
H.X. Pang, Y.H. Hui, K. Fan, X.J. Xing, Y. Wu, J.H. Yang, W. Shi, Z.F. Xie, Chin. Chem. Lett. 27, 335 (2012)
D. Kumar, M. Sonawane, B. Pujala, V.K. Jain, S. Bhagat, A.K. Chakraborti, Green Chem. 15, 2872 (2013)
Acknowledgements
We are grateful to the head of the Department of Chemistry, Dr. B.A.M., University, Aurangabad, 431004 (MS), India for providing the laboratory facility. The corresponding author MKL is thankful to UGC New Delhi (INDIA) for providing grant under major research Project Funding No. 43-184/2014 (SR). The authors are thankful to STIC Cochin and SAIF Chandigarh, IIT Madras (Department of Chemistry), CDRI Lucknow characterization facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gadekar, S.P., Lande, M.K. TS-1 zeolite as a Lewis acid catalyst for solvent-free one-pot synthesis of 1,3-thiazolidin-4-ones. Res Chem Intermed 45, 237–247 (2019). https://doi.org/10.1007/s11164-018-3599-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3599-2