Abstract
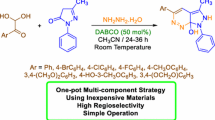
A simple, efficient, and eco-friendly protocol has been developed for the synthesis of novel 3-((benzo[d]thiazol-2-ylamino)(4-methoxyphenyl)methyl)-4-hydroxy-1-methylquinolin-2(1H)-one derivatives using a one-pot C–C and C–N bond forming strategy from the reaction of 4-hydroxy-1-methylquinolin-2(1H)-one, 2-aminobenzothiazole and aromatic aldehydes in aqueous solvent without using any metal catalyst. Several advantages of this protocol include its operational simplicity, short reaction time, mild reaction condition, efficient utilization of all the reactants, wide functional group tolerance, using water as an environmentally friendly solvent and non-chromatographic purification procedure.
Graphic abstract





Similar content being viewed by others
References
T.C. Küehler, J. Fryklund, N.-K. Bergman, J. Weilitz, A. Lee, H. Larsson, J. Med. Chem. 38, 4906 (1995)
P. Madsen, L.B. Knudsen, F.C. Wiberg, R.D. Carr, J. Med. Chem. 41, 5150 (1998)
L. Llauger, H.Z. He, J. Kim, J. Aguirre, Z. Rosen, U. Peters, P. Davies, G. Chiosis, J. Med. Chem. 48, 2892 (2005)
N. Pietrancosta, A. Moumen, R. Dono, P. Lingor, V. Planchamp, F. Lamballe, M. Bahr, J.L. Kraus, F. Maina, J. Med. Chem. 49, 3645 (2006)
R. Gali, J. Banothu, M. Porika, R. Velpula, S. Hnamte, Bioorg. Med. Chem. Lett. 24(17), 4242 (2014)
R. Gupta, R.P. Chaudhary, J. Mol. Struct. 189, 1049 (2013)
N. Pietrancosta, A. Moumen, R. Dono, P. Lingor, V. Planchamp, M. Lamballe, M. Ba¨hr, J.-L. Kraus and F. Maina. J. Med. Chem. 12, 3645, (2006)
A. Patel, S.Y. Sharp, K. Hall, W. Lewis, M.F. Stevens, P. Workman, C.J. Moody, Org. Biomol. Chem. 14, 3889 (2016)
T.H. Al-Tel, R.A. Al-Qawasmeh, R. Zaarour, Eur. J. Med. Chem. 46, 1881 (2011)
S.B. Srinivasa, B. Poojary, U. Brahmavara, A.J. Das, S.K. Middha, Chem. Sel. 3, 12485 (2018)
J. Zhao, H. Huang, W. Wu, H. Chen, H. Jiang, Org. Lett. 15, 2604 (2013)
O. Afzal, S. Kumar, M.R. Haider, M.R. Ali, R. Kumar, M. Jaggi, S. Bawa, Eur. J. Med. Chem. 07, 044 (2014)
O.I. El-Sabbagh, M.M. Baraka, S.M. Ibrahim, C. Pannecouque, G. Andrei, R. Snoeck, J. Balzarini, A.A. Rashad, Eur. J. Med. Chem. 44, 3753 (2009)
V. Zaharia, A. Ignat, N. Palibroda, B. Ngameni, V. Kuete, C.N. Fokunang, M.L. Moungang, B.T. Ngadjui, Eur. J. Med. Chem. 45, 5085 (2010)
I. Hutchinson, S.A. Jennings, B.R. Vishnuvajjala, A.D. Westwell, M.F.G. Stevens, J. Med. Chem. 45, 747 (2002)
A. Kamal, M.N.A. Khan, K.S. Reddy, K. Rohini, Bioorg. Med. Chem. 15, 1013 (2007)
R.B. Pathak, B. Jahan, S.C. Bahel, Bokin Bobai. 9, 480 (1981)
D.D. Erol, U. Çaliş, R. Demirdamar, N. Yuluĝ, M. Ertan, J. Pharm. Sci. 84, 465 (1995)
F. Azam, B.A. El-gnidi, I.A. Alkskas, M.A. Ahmed, Med. Chem. 25, 826 (2010)
Y. Kumar, R. Green, D.S. Wise, L.L. Wotring, L.B. Townsend, J. Med. Chem. 36, 3852 (1993)
R.N. Sharma, F.P. Xavier, K.K. Vasu, S.C. Chaturvedi, S.S. Pancholi, Med. Chem. 24, 897 (2009)
M.U. Rahman, G. Jeyabalan, P. Saraswat, G. Parveen, S. Khan, M.S. Yar, Synth. Commun. 47(5), 408 (2016)
D. Monchaud, C. Allain, M.P. Teulade-Fichou, Bioorg. Med. Chem. Lett. 26(18), 4845 (2016)
X. Chen, Y. Du, H. Sun, F. Wang, L. Kong, M. Sun, Bioorg. Med. Chem. Lett. 24(3), 887 (2014)
M. Zahedifard, F.L. Faraj, M. Paydar et al., Curr. Pharm. Des. 21(23), 3426 (2015)
V. Chandregowda, AK. Kush, G. Chandrasekara Reddy. Eur. J. Med. Chem. 44 (7), 3055, (2014).
V. Alagarsamy, V. Raja Solomon, K. Dhanabal. Bioorg. Med. Chem. 15(1), 241, (2007).
P. Nandy, M.T. Vishalakshi, A.R. Bhat, Indian. J. Heterocycl. Chem. 15(3), 294 (2006)
H. Georgey, N. Abdel-Gawad, S. Abbas, Molecules 13(10), 2569 (2008)
P. Verhaeghe, N. Azas, M. Gasquet, Bioorg. Med. Chem. Lett. 18(1), 401 (2008)
M, A. Ismail, S. Barker, D. A. Abau el-Ella, K. A. Abouzid, R. A. Toubar, M. H. Todd. J. Med. Chem. 49 (5), 1535, (2006).
M.S. Malamas, J. Millen, J. Med. Chem. 34(4), 1503 (1991)
M.P.D. Mahindaratne, K. Wimalsena, J. Org. Chem. 3, 2866 (1998)
M. Zhang, Y.H. Liua, Z.R. Shanga, H.C. Hu, Z.H. Zhang, Catal. Commun. 88, 44 (2017)
M.N. Chen, L.P. Mo, Z.S. Cui, Z.H. Zhang, Curr. Opin. Green Sustain. Chem. 15, 37 (2019)
A. R. Khosropour, M. M. Khodaei, H. Moghannian, Synlett, 955, (2005).
V. Anuradha, P.V. Srinivas, P. Aparna, M.J. Rao, Tetrahedron Lett. 47, 4935 (2006)
T. Li, Y. Souma, O. Xu, Catal. Today 111, 291 (2006)
K.B. Wang, G.Y. Wang, Chin. Chem. Lett. 18, 813 (2007)
J.D. Mosely, Tetrahedron Lett. 46, 3181 (2005)
B.B. Thompson, M.J. Enone, Org. Lett. 13, 3289 (2011)
B.M. Trost, A.J. Frontier, J. Am. Chem. Soc. 122, 11727 (2000)
B.M. Trost, A.C. Gutierrez, R.C. Livingston, Org. Lett. 11, 2539 (2009)
B. Jiang, S.J. Tu, P. Kaur, W. Wever, G. Li, J. Am. Chem. Soc. 131, 11660 (2009)
B. Jiang, M.S. Yi, F. Shi, S.J. Tu, S. Pindi, P. McDowell, G. Li, Chem. Commun. 48, 808 (2012)
M. Li, H. Cao, Y. Wang, X.L. Lv, L.R. Wen, Org. Lett. 14, 3470 (2012)
Y. Coquerel, T. Boddaert, M. Presset, D. Mailhol, J. Rodriguez, Wiley-VCH. Weinheim, Germany 9, 187 (2010)
S. Tiwariand, A. Kumar, Angew. Chem. Int. Ed. 45, 4824 (2006)
S.G. Balwe, Y.T. Jeong, RSC Adv. 6, 107232 (2016)
S.G. Balwe, K.T. Lim, B.G. Cho, Y.T. Jeong, Tetrahedron 73, 3564 (2017)
S.G. Balwe, V.V. Shinde, A.A. Rokade, S.S. Park, Y.T. Jeong, Catal. Commun. 99, 126 (2017)
Y.T. Jeong, S.G. Balwe, Org. Chem. Front. 5, 1632 (2018)
A.M. Jadhav, S.G. Balwe, K.T. Lim, Y.T. Jeong, Tetrahedron 73, 2813 (2017)
A.M. Jadhav, S.G. Balwe, J.S. Kim, K.T. Lim, Y.T. Jeong, Tetrahedron Lett. 60, 565 (2019)
S.G. Balwe, A.A. Rokade, S.S. Park, Y.T. Jeong, Catal. Commun. 128, 105703 (2019)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yadav, M.B., Vagh, S.S. & Jeong, Y.T. A novel synthesis of 3-((benzo[d]thiazol-2-ylamino)(phenyl)methyl)-4-hydroxy-1-methylquinolin-2(1H)-one via consecutive C–C and C–N bond formation in water. Res Chem Intermed 46, 3801–3815 (2020). https://doi.org/10.1007/s11164-020-04173-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04173-0