Abstract
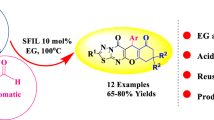
Herein, we report a catalyst-free, one-pot three-component reaction of 5-amino-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidin-7-one, aromatic aldehyde, and dimedone in ethylene glycol as a green solvent at 100 °C for the easy access of hexahydro-5H-thiazolo[2',3':2,3]pyrimido[4,5-b]quinoline. Catalyst-free, green solvent, simple procedure, mild reaction conditions, easy work-up procedure, and good to excellent yields are the significant advantages of this protocol.
Graphical abstract



Similar content being viewed by others
References
Joule JA, Mills K (2000) Heterocyclic chemistry, 4th edn. Wiley-Blackwell, Oxford
Katritzky AR (ed) (2008) Comprehensive heterocyclic chemistry III, vol 4–6. Elsevier, Amsterdam
Yang D, An B, Wei W, Tian L, Huang B, Wang H (2015) Copper-catalyzed domino synthesis of nitrogen heterocycle-fused benzoimidazole and 1,2,4- benzothiadiazine 1,1-dioxide derivatives. ACS Comb Sci 17:113–119
Eicher T, Hauptmann S (2003) The chemistry of heterocycles Structure, reactions, syntheses, and applications. Wiley, Weinheim
Joule JA, Mills K (2000) Heterocyclic chemistry, 4th edn. Blackwell, Oxford
Batool I, Saeed A, Qureshi IZ, Kalsoom S, Razzaq A (2016) Synthesis, molecular docking and biological evaluation of new thiazolopyrimidine carboxylates as potential antidiabetic and antibacterial agents. Res Chem Intermed 42:1139–1163. https://doi.org/10.1007/s11164-015-2078-2
Banoth S, Boda S, Perugu S, Balabadra S, Manga V (2017) Design, synthesis, biological evaluation and in silico molecular docking studies of novel benzochromeno [2, 3-d] thiazolopyrimidine derivatives. Res Chem Intermed 44:1833–1846. https://doi.org/10.1007/s11164-017-3201-3
Yousif MNM, El-Sayed WA, Abbas HAS, Awad HM, Yousif NM (2017) Anticancer activity of new substituted pyrimidines, their thioglycosides and thiazolopyrimidine derivatives. Appl. Pharm. Sci 7:21–32. https://doi.org/10.7324/JAPS.2017.71104
Youssef AMS, Fouda AM, Farty RM (2018) Microwave assisted synthesis of some new thiazolopyrimidine and pyrimidothiazolopyrimidopyrimidine derivatives with potential antimicrobial activity. Chem Cent J 12:50–63. https://doi.org/10.1186/s13065-018-0419-0
Al-Rashood ST, Elshahawy SS, El-Qaias AM, El-Behedy DS, Hassanin AA, El-Sayed SM, El-Messery SM, Shaldam MA, Hassan GS (2020) New thiazolopyrimidine as anticancer agents: synthesis, biological evaluation, DNA binding, molecular modeling and ADMET study. Bioorg Med Chem Lett 30:127611–127627. https://doi.org/10.1016/j.bmcl.2020.127611
Istanbullu H, Bayraktar G, Akbaba H, Cavus I, Coban G, Butuner BD, Kilimcioglu AA, Ozbilgin A, Alptuzun V, Erciyas E (2020) Design, synthesis, and in vitro biological evaluation of novel thiazolopyrimidine derivatives as antileishmanial compounds. Arch Pharm. https://doi.org/10.1002/ardp.201900325
Gholami M, Youseftabar-Miri L, Askarizadeh E, Hosseinjani-Pirdehi H (2021) A concise, facile and MCR-GAP chemistry strategy for the synthesis of spiro[benzo[4,5]thiazolo[3,2-a]pyrano[2,3-d]pyrimidine-4,3′-indoline] derivatives as fluorescent cellular imaging agents. J Mol Struct 1245:131044. https://doi.org/10.1016/j.molstruc.2021.131044
Nemr MTM, AboulMagd AM (2020) New fused pyrimidine derivatives with anticancer activity: synthesis, topoisomerase II inhibition, apoptotic inducing activity and molecular modeling study. Bioorg Chem 103:104134. https://doi.org/10.1016/j.bioorg.2020.104134
Sekhar T, Thriveni P, Venkateswarlu A, Daveedu T, Peddanna K, Sainath SB (2020) One-pot synthesis of thiazolo[3,2-a]pyrimidine derivatives, their cytotoxic evaluation and molecular docking studies. Spectrochim Acta A Mol Biomol Spectrosc 231:118056. https://doi.org/10.1016/j.saa.2020.118056
Basiony EA, Hassan AA, Al-Amshany ZM, Abd-Rabou AA, Abdel-Rahman AAH, Hassan NA, El-Sayed WA (2020) Synthesis and cytotoxic activity of new thiazolopyrimidine sugar hydrazones and their derived acyclic nucleoside analogues. Molecules 25:399. https://doi.org/10.3390/molecules25020399
Ackova DG, Kotur-Stevuljevic J, Mishra CB, Luthra PM, Saso L (2019) Antioxidant properties of synthesized bicyclic thiazolopyrimidine derivatives as possible therapeutic agents. Appl Sci 9:113–122. https://doi.org/10.3390/app9010113
Youssef MM, Amin MA (2012) Microwave assisted synthesis of some new thiazolopyrimidine, thiazolodipyrimidine and thiazolopyrimidothiazolopyrimidine derivatives with potential antioxidant and antimicrobial activity. Molecules 17:9652–9667. https://doi.org/10.3390/molecules17089652
Rashad AE, Shamroukh AH, Abdel-Megeid RE, El-Sayed WA (2010) Synthesis, reactions and antimicrobial evaluation of some polycondensedthieno-pyrimidine derivatives. Synth Commun 40:1149–1160. https://doi.org/10.1080/00397910903050954
El-Emary TI, Abdel-Mohsen SA (2006) Synthesis and antimicrobial activity of some new1,3-diphenylpyrazoles bearing pyrimidine, Pyrimidinethione, thiazolopyrimidine, triazolopyrimidine, thio- and alkylthiotriazolopyrimidinone moieties at the 4-position. Phosphorus Sulfur Silicon Relat Elem 181:2459–2474. https://doi.org/10.1080/10426500600754695
Maddila S, Damu GLV, Oseghe EO, Abafe OA, Venakata RC, Lavanya P (2012) Synthesis and biological studies of novel biphenyl-3,5-dihydro-2H-thiazolo-pyrimidines derivatives. J Korean Chem Soc 56:334–340. https://doi.org/10.5012/jkcs.2012.56.3.334
Flefel EE, Salama MA, El-Shahat M, El-Hashash MA, El-Farargy AF (2007) A novel synthesis of some new pyrimidine and thiazolopyrimidine derivatives for anticancer evaluation. Phosphorus Sulfur Silicon Relat Elem 182:1739–1756. https://doi.org/10.1080/10426500701313912
Al-Omary FA, Hassan GS, El-Messery SM, ElSubbagh HI (2012) Substituted thiazoles V. Synthesis and antitumor activity of novel thiazolo[2,3-b]quinazoline and pyrido[4,3-d] thiazolo[3,2-a] pyrimidine analogues. Eur J Med Chem 47:65–72. https://doi.org/10.1016/j.ejmech.2011.10.023
Amr AEG, Maigali SS, Abdulla MM (2008) Synthesis, and analgesic and antiparkinsonian activities of thiopyrimidine, pyrane, pyrazoline, and thiazolopyrimidine derivatives from 2-chloro-6-ethoxy- 4-acetylpyridine. Mon Chem 139:1409–1415. https://doi.org/10.1007/s00706-008-0937-x
Cai D, Zhang ZH, Chen Y, Yan XJ, Zou LJ, Wang YX, Liu XQ (2015) Synthesis, antibacterial and antitubercular activities of some 5H-thiazolo[3,2-a]pyrimidin-5-ones and sulfonic acid derivative. Molecules 20:16419–16434. https://doi.org/10.3390/molecules200916419
Said M, Abouzid K, Mouneer A, Ahmedy A, Osman AM (2004) Synthesis and biological evaluation of new thiazolopyrimidines. Arch Pharm Res 27:471–477. https://doi.org/10.1007/BF02980118
Branstetter BJ, Breitenbucher JG, Lebsack AD, Xiao W (2008) Thiazolopyrimidine Modulators of TRPV1. U.S. Patent WO 005303, 10 January
Duval R, Kolb S, Braud E, Genest D, Garbay C (2009) Rapid discovery of triazolobenzylidenethiazolopyrimidines (TBTP) as CDC25 phosphatase inhibitors by parallel click chemistry and in situ screening. J Comb Chem 11:947–950. https://doi.org/10.1021/cc900140f
Kolb S, Mondésert O, Goddard ML, Jullien D, Villoutreix BO, Ducommun B, Garbay C, Braud E (2009) Development of novel thiazolopyrimidines as CDC25B phosphatase inhibitors. Chem Med Chem 4:633–648. https://doi.org/10.1002/cmdc.200800415
Mahgoub MY, Elmaghraby AM, Harb AA, da Silva JLF, Justino GC, Marques MM (2019) Synthesis, crystal structure, and biological evaluation of fused thiazolo[3,2-a]pyrimidines as new acetylcholinesterase inhibitors. Molecules 24:2306. https://doi.org/10.3390/molecules24122306
Liu SJ, Yang L, Jin Z, Huang EF, Wan DCC, Lin HQ, Hu C (2009) Design, synthesis, and biological evaluation of 7H-thiazolo[3,2-b]- 1,2,4-triazin-7-one derivatives as novel acetylcholinesterase inhibitors. ARKIVOC 10:333–348. https://doi.org/10.2174/157018010789869343
Mohamed SF, Flefel EM, Amr AEGE, Abd El-Shafy DN (2010) Anti-HSV-1 activity and mechanism of action of some new synthesized substituted pyrimidine, thiopyrimidine and thiazolopyrimidine derivatives. Eur J Med Chem 45:1494. https://doi.org/10.1016/j.ejmech.2009.12.057
Flefel EE, Salama MA, El-Shahat M, El-Hashash MA, El-Farargy AF (2007) A novel synthesis of some new pyrimidine and thiazolopyrimidine derivatives for anticancer evaluation. Phosphorus Sulfur Silicon Relat Elem 182:1739. https://doi.org/10.1080/10426500701313912
Valente S, Mellini P, Spallotta F, Carafa V, Nebbioso A, Polletta L, Carnevale I, Saladini S, Trisciuoglio D, Gabellini C, Tardugno M, Zwergel C, Cencioni C, Atlante S, Moniot S, Steegborn C, Budriesi R, Tafani M, Bufalo DD, Altucci L, Gaetano C, Mai A (2016) 1,4-Dihydropyridines active on the SIRT1/AMPK pathway ameliorate skin repair and mitochondrial function and exhibit inhibition of proliferation in cancer cells. J Med Chem 59:1471–1491. https://doi.org/10.1021/acs.jmedchem.5b01117
Briede J, Stivrina M, Vigante B, Stoldere D, Duburs G (2008) Acute effect of antidiabetic 1,4-dihydropyridine compound cerebrocrast on cardiac function and glucose metabolism in the isolated, perfused normal rat heart. Cell Biochem Funct 26:238–245. https://doi.org/10.1002/cbf.1442
Kumar A, Sharma S, Tripathi VD, Maurya RA, Srivastava SP, Bhatia G, Tamrakar AK, Srivastava AK (2010) Design and synthesis of 2, 4-disubstituted polyhydroquinolines as prospective antihyperglycemic and lipid modulating agents. Bioorg Med Chem 18:4138–4148. https://doi.org/10.1016/j.bmc.2009.11.061
Pontremoli R, Leoncini G, Parodi A (2005) Use of nifedipine in the treatment of hypertension. Expert Rev Cardiovasc Ther 3:43–50. https://doi.org/10.1586/14779072.3.1.43
Bossert F, Meyer H, Wehinger E (1981) 4-Aryldihydropyridines, a new class of highly active calcium antagonists. Angew Chem Int Ed Engl 20:762–769. https://doi.org/10.1002/anie.198107621
Love B, Goodman M, Snader K, Tedeschi R, Macko E (1974) Hantzsch-type dihydropyridine hypotensive agents. J Med Chem 17:956–965. https://doi.org/10.1021/jm00255a010
Triggle DJ (2003) 1,4-Dihydropyridines as calcium channel ligands and privileged structures. Cell Mol Neurobiol 23:293–303. https://doi.org/10.1023/A:1023632419813
El-Ashmawy MB, El-Sherbeny MA, El-Gohary NS (2013) Synthesis and antitumor screening of new series of pyrimido-[4,5- b]quinolines and [1,2,4]triazolo[20,30:3,4]pyrimido[6,5-b]quinolines. Med Chem Res 22:2724–2736. https://doi.org/10.1007/s00044-012-0272-y
Abbas HAS, Hafez HN, El-Gazzar ARBA (2011) Synthesis, in vitro antimicrobial and in vivo antitumor evaluation of novel pyrimidoquinolines and its nucleoside derivatives. Eur J Med Chem 46:21–23. https://doi.org/10.1016/j.ejmech.2010.09.071
Marco-Contelles J, Leon R, Rios C, Samadi A, Andrisano V, Huertas O, Barril X, Luque FJ, Rodriguez-Franco MI, Lopez B, Lopez MG, Garcia AG, Carreiras C, Villarroya M (2009) Tacripyrines, the first tacrine−dihydropyridine hybrids, as multitarget-directed ligands for the treatment of Alzheimer’s disease. J Med Chem 52:2724–2732. https://doi.org/10.1021/jm801292b
Zhang D, Wu LZ, Zhou L, Han X, Yang QZ, Zhang LP, Tung CH (2004) Photocatalytic hydrogen production from hantzsch 1,4-dihydropyridines by platinum (II) terpyridyl complexes in homogeneous solution. J Am Chem Soc 126:3440–3441. https://doi.org/10.1021/ja037631o
Jannati S, Esmaeili AA (2018) Synthesis of novel spiro[benzo[4,5]thiazolo[3,2-a]chromeno[2,3-d]pyrimidine-14,3′-indoline]-1,2′,13(2H)-triones via three component reaction. Tetrahedron 74:2967–2972. https://doi.org/10.1016/j.tet.2018.04.092
Esmaeilinezhad M, Esmaeili AA, Jannati S (2018) Facile construction of novel fused chromeno[2,3-d]thiazolo[3,2-a]pyrimidine derivatives in biocompatible ionic liquid under solvent-free conditions. J. Chem. Res 42:618–622. https://doi.org/10.3184/174751918X15423512867751
Acknowledgements
The Ferdowsi University of Mashhad Research department is acknowledged for financial support (Grant No. 3/48721).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tabibi, T., Esmaeili, A.A. Efficient and green synthesis of novel hexahydro-5H-thiazolo[2',3':2,3]pyrimido[4,5-b]quinoline derivatives. Mol Divers 27, 477–486 (2023). https://doi.org/10.1007/s11030-022-10439-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10439-z