Abstract
Between 2009 and 2021 almost the complete beam trawl fleet of the Netherlands switched from conventional beam trawls (BT) to pulse trawls (PT) using electrical stimulation to catch sole, Solea solea. Electric fishing, being banned in the EU in 1988, was made possible in 2006 under a derogation. Over the years stakeholders expressed concern about ecosystem effects. Here we review the research conducted. PT improved the selectivity of the fishery and reduced the ecological side effects. PT caught more sole per hour fishing but less discards and benthos than BT. The transition to PT reduced the surface area swept (lower towing speed), sediment depth of disturbance and associated benthic impacts, as well as fuel consumption. Laboratory experiments with 9 fish and 17 benthic invertebrate species showed that exposure to a commercial bipolar pulse stimulus did not result in harmful effects except in cod. Autopsy of cod sampled from PT revealed that 40% had an internal injury. Injury rates in other roundfish species was low (< 2%) and absent in flatfish. Electrical-induced impacts on biogeochemistry were not observed. The transition increased competition with other fishers which fed the resentment against PT. Governance arrangements under which the number of temporary licenses expanded, undermined legitimacy of the gear, resulting in a ban in 2021. Although questions about the ecological impact of electrical stimulation remain, adverse effects are considered negligible in comparison with the benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mobile bottom-contacting fishing gears mechanically stimulate fish out of, or from, the seafloor into the net. This mechanical stimulation physically disturbs the seafloor and affects benthic habitats and associated communities (Collie et al. 2000; Sciberras et al. 2018) and biogeochemical processes (Pusceddu et al. 2005, Oberle et al. 2016, van de Velde et al. 2018). The benthic impact of bottom trawls and dredges is related to the physical disturbance by the gear components (Eigaard et al. 2016), in particular their penetration depth and associated mortality imposed (Hiddink et al. 2017), and the sensitivity of the marine biota (Rijnsdorp et al. 2018a, Sciberras et al. 2018, Hiddink et al. 2019). In addition, these gears are often unselective, particularly when using small mesh sizes, resulting in a substantial bycatch of small fish, benthic invertebrates and other marine species (Kelleher 2005; Gray and Kennelly 2018; Uhlman et al. 2019) and have a high fuel consumption and CO2 emission (Tyedmers et al. 2005; Parker and Tyedmers 2015; Sala et al. 2022).
Replacing mechanical stimulation by electrical stimulation has the potential to improve the ecological sustainability of bottom trawl fisheries by reducing adverse ecological effects (Soetaert 2015; McConnaughey et al. 2020). Electrical stimulation may be used to induce a startle response in bottom dwelling or benthic organisms to drive them out of the sea bed and facilitate their catch without the need to deploy a heavy groundrope or tickler chains. Alternatively, it may be used to induce a cramp response to immobilize organisms that can no longer escape from the fishing gear. Because the sensitivity to an electric stimulus increases with body size, electrical stimulation may in theory be used to improve the size selectivity of the gear. Electrical stimulation has long been used in freshwater fishing where it proved to be an efficient method (Snyder 2003b, Rytwinski et al. 2019). The application in marine fisheries has been developed much later as it is technically challenging to develop safe and robust pulse generators that can be used in salt water on board of commercial fishing vessels (van Marlen and de Haan 1988). Electrotrawls have in the past two decades become available for the fishery for sole (Solea solea), shrimps (Crangon crangon) and razor clams (Ensis spp) (review in Soetaert 2015).
Although promising, the use of electricity in capture fisheries may also have adverse effects, as it is well-documented in freshwater electrofishing that fish may suffer from internal injuries such as haemorrhages and spinal fractures (Snyder 2003b). The concern about possible adverse effects of electricity on marine biota and fear for overexploitation has motivated a ban of electrotrawls in several countries. In China, for example, electrical stimulation has been banned in the shrimp fishery following unregulated growth in fishing effort of electrotrawls (Yu et al. 2007). In the European Union (EU), fishing methods using electrical stimulation were prohibited in 1988 (Linnane et al. 2000; Haasnoot et al. 2016).
Despite the ban of electrotrawling in the EU, research into use of electricity in marine fisheries continued in the early 1990s. Results showed potential application in the beam trawl fishery for brown shrimps and for the beam trawl fishery for sole (Soetaert 2015). When in the 2000s the beam trawl fishery for sole was faced with high fuel costs, poor economic performance, decreasing fish quota and the increased societal concern about adverse ecological side effects, electrotrawls were seen as a promising alternative that could improve the economic and ecologic sustainability of the fishery (van Balsfoort et al. 2006; van Hoof et al. 2020). In line with the objective of the Common Fisheries Policy to support technological innovations to reduce adverse side effects of fishing gears, a derogation to the ban on electrotrawling in the North Sea was agreed in 2006, allowing 5% of the beam trawl fleet of each Member State (ICES areas IVc and IVb) to use the electrical pulse trawl with accompanying research. The derogation for the Dutch fleet was expanded in later years (Haasnoot et al. 2016) but the ban was reinstated in 2019 and took full effect in 2021 (Delaney et al. 2023).
The objective of this paper is to review the research carried out during the pulse trawling period (Fig. 1) and update the analysis of catch and effort data sets to assess whether a transition from the conventional tickler beam trawl (BT) to the pulse trawl (PT) could improve the sustainability of the sole fishery. The paper starts with (i) a description of the governance arrangements that allowed beam trawl vessels to switch to pulse trawling, followed by (ii) a description of the beam trawl fishery for sole including a review of efficiency and selectivity of both gears, and a review of (iii) laboratory studies on the effect of pulse stimulation on marine biota and the biogeochemical processes; (iv) field experiments on the impact of the PT and BT gear on the sea floor and benthic ecosystem; (v) fleet-level impact studies on the implications of the transition on the change in the surface area trawled, discard production, occurrence of pulse-induced injuries, benthic impact and sediment resuspension; (vi) socio-economic consequences including effects on other fisheries. Finally the available scientific evidence is synthesized to assess whether the transition to PT can improve the sustainability of the beam trawl fishery for sole.
Timeline of pulse trawl development in the Netherlands (adapted from Delaney et al. 2023)
Governance
In the 1990s, high fuel costs, decreasing fish quota and increased societal concern about the impact of trawling jeopardized the viability of the beam trawl fisheries (van Balsfoort et al. 2006; van Hoof et al. 2020). A Societal Covenant was agreed in the Netherlands between the government, the fishing industry and NGOs with a pathway to a transition process towards sustainable North Sea fisheries.Footnote 1 As part of this transition, research into the use of electricity to catch flatfish was stimulated. Results were promising but with the EU ban of 1988 on the use of electricity in fishing gears, institutionally this innovation was blocked. The Dutch ministry was willing to open up discussions to change the rules but first wished to see whether or not electrical pulse trawling had potential by applying it to a fishing vessel, as until then all research had taken place on research vessels (Haasnoot et al. 2016). The PT was first tested on a commercial vessel in 2004. A Pulse Steering Group (PSG), comprising representatives from the industry, NGOs, the ministry and scientists, was set up to guide the process. When results of the test were promising, discussions on the regulation were opened. This happened at a time where the EU Commission stimulated research into sustainable fishing techniques (EC 2004). In 2005 the EU commission indicated that it would be possible to further expand the introduction of PT, if scientific advice would be positive (Haasnoot et al. 2016). Both the International Council for the Exploration of the Sea (ICES) and the EU’s own Scientific, Technical and Economic Committee on Fisheries (STECF) provided cautiously positive, precautionary advice (ICES 2006; STECF 2006). Subsequently, the EU Commission decided in 2006 to permit 5% of Member States’ fishing vessels in the North Sea to use the PT, under a derogation (Fig. 1).
In 2008 an investment scheme (funded by the EU) was made available in the Netherlands under the new fisheries innovation framework for 5 vessels to acquire the PT gear (Haasnoot et al. 2016). Within a few years, through active knowledge sharing, these vessels collectively worked out how to fish with PT gear.Footnote 2 Their experiences were guided by research and shared with the wider sector. Although catchability of marketable sole and plaice was initially lower than with the BT, the fuel cost reduction made it more profitable (Poos et al. 2020). In 2010 more vessels applied for a license under the 5%-derogation (Haasnoot et al. 2016). When the Netherlands hit the 5% derogation ceiling set under European legislation in 2010, the fishing industry asked to allow more vessels to fish with PT. This call was pushed by the economic situation in the Dutch beam-trawl fleet, where vessels who switched to PT were once again making net profits, as opposed to companies who still used traditional BT (Turenhout et al. 2016). Accommodating the wish from the Dutch fishing industry to maintain a level playing field within the Dutch fleet, and with support of Dutch NGOs, the Dutch government negotiated an increase of the number of (temporary) licenses with the European Commission in 2011 (Haasnoot et al. 2016). This was based on article 43, 850/1998; a regulation for the conservation of fishery resources through technical measures for the protection of juveniles of marine organisms (EC 1998). The Netherlands could hand out another 20 licences (to 42) and committed to monitor the bycatch of the pulse trawlers via self-sampling.
With this expansion of the fleet, concern outside of the Netherlands grew. In response, the PSG organised meetings in Belgium, Germany and the UK to provide information on the PT to fishers. This was done in addition to regular updates in the North Sea Advisory Commission (NSAC) (Haasnoot et al. 2016). In this time period the Dutch government and industry strongly pushed the PT as the solution to the problems associated with beam trawling. This resulted in a pushback response from other countries (Haasnoot et al. 2016). It is important to note that the ‘pulse solution’ was mostly relevant for the Dutch fleet, as (pre-Brexit) the Netherlands were annually allocated 74% of sole EU’s North Sea sole TAC. In addition, sole quota from other North Sea Member States are often in the hands of Dutch companies who fly the flag of these Member States (most notably the UK). Through this so-called quota hopping (Morin 2000; Hatcher et al. 2002), the ‘Dutch EU share’ of sole increased to about 90% (pre-Brexit). Despite the pushback from other Member States, the Netherlands continued to adopt the new gear in management plans (i.e. agreements on fishing in N2000 areas) and sought a permanent admission of the gear in EU legislation or else expansion of the derogation. These efforts exacerbated resistance from Member States and the EU Commission (Haasnoot et al. 2016). However as more Dutch fishers requested to be allowed to fish with PT, due to the better economic performance, the Netherlands continued seeking ways to expand. During a meeting between the Dutch government and the Commissioner of DGMARE in 2014, it was agreed to expand the PT fleet again, this time linked to the landing obligation that was established in the Common Fisheries Policy reform of 2013 (EC 2013). Now, the number of pulse licenses increased to a total number of 84. Both the 2011 and 2014 license expansions on top of the original 5% derogation, were approved by the EU Commission under the condition that research into the ecological effects of pulse trawling was carried out. On top of ongoing research, the Netherlands prepared a comprehensive multi-annual impact assessment project (IAPF) that started in 2016 and ended in 2020 (Fig. 1).
Stakeholder involvement
During the initial development phase (between 2004 and 2011) stakeholders had been involved right from the start. However, this mainly took place within the Netherlands. Dutch NGOs were already aligned via the Societal Covenant. Dutch fishers were informed of the collective progress made by the group of 5 pioneers (Haasnoot et al. 2016; Delaney et al. 2023). International stakeholders were not specifically addressed, only on an ad hoc basis and mostly via the NSAC (Haasnoot et al. 2016). It is only after 2014 that deliberate international stakeholder engagement was sought. This was mostly in response to criticism from other Member States that had been building up over a couple of years over the way the Dutch government had arranged extra pulse licences (bilaterally with the commission). The push for EU acceptance of the gear (looking for all kinds of possibilities in the legislation—see Haasnoot et al. 2016) had alienated many EU stakeholders, which were upset about the way these licences had been ‘arranged’ and the size of the pulse fleet. They were also concerned about the control and enforcement system in use, the ecosystem impacts and the socio-economic consequences of the pulse for other fishes. Fishers not using PT were out-competed or witnessed pulse fishers arriving at ‘softer’ grounds where beam trawlers had never been seen before (Kraan et al. 2015; Haasnoot et al. 2016). When the extent of these stakeholder concerns became clear in 2015 (Kraan et al. 2015), the Dutch government started organising so-called International Stakeholder Dialogue Meetings (2015, 2017 and 2018) in conjunction to the multi-annual research project IAPF (Steins et al. 2017; Kraan and Schadeberg 2018). Stakeholders involved in those meetings indicated later in an evaluation that although they appreciated the engagement as effective, the engagement had been sought too late in the innovation process (Delaney et al. 2023). In the midst of this all, in 2016, a French NGO Bloom started a campaign against PT. Opponents compared pulse trawling to scientific whaling and claimed that PT would turn fishing grounds into a graveyard by electrocuting marine life in its wake and that it would outcompete other, most notably small-scale, fishers (Bloom 2018; Le Manach et al. 2019). Bloom’s campaign, in joint forces with small scale fishers and other NGOs was a mix of political lobbying; mobilizing different groups in society (i.e. chefs); petitioning; law suits, requests for infringement procedures and a simple clear message (no to the electrocution of fish and the desertification of the ocean).Footnote 3 It resulted in extensive media exposure and proved to be influential.Footnote 4
Decision making process EU
Following the 2013 reform of the Common fisheries Policy (EC 2013), it was necessary to revise the technical conservation measures for fisheries.Footnote 5 As the ban on using electricity in marine fisheries was laid down in the technical measures regulations, the discussion on lifting the ban on PT was part of this revision. Where initially in 2017 the fisheries committee of the European Parliament proposed to allow PT under the revised technical measures if research showed positive results about the gear’s impact, a year later the Parliament voted for a ban on PT. A crucial aspect in this process was that the rapporteur of the fisheries committee, the Spanish MEP Mato, in 2017 did not get the mandate to directly negotiate, on behalf of the Parliament, with the EU Commission and Council in the so-called trilogue that is part of the legislative process. Instead, the matter was scheduled for a plenary vote in Parliament in January 2018.Footnote 6 The 750 European parliamentarians were lobbied by proponents and opponents of PTFootnote 7 prior to the vote, which ended with 402 in favor of a full ban. A year later, on February 2019 it was decided in trilogue that PT will be banned from July 1, 2021. The trilogue decision did not await the final results of the multi-annual research programme (IAPF, (Rijnsdorp et al. 2020a)) and subsequent advice by ICES (ICES 2020b). From then onwards pulse fishing is only allowed in the context of research (article 25, Regulation 2019/1241) and only after both ICES and STECF agree on a scientific protocol and research plan (Penca 2022). As a consequence, the Dutch vessels equipped with a PT had to revert back to traditional BT.
Court of justice
Three months after the decision of the EU (April 2019), the Netherlands filed a complaint at the European Court of Justice against the EU Council and the Parliament (Case C-733/19) (Penca 2022). The Netherlands argued that the ban was not based on the best available scientific advice, and therefore should be overturned. In 2021 the Court rejected the complaint and request, stating that whilst the science available at the time of the decision recognized advantages of PT over BT, as yet not all risks associated with the gear had been identified. The Court also ruled that under the Common Fisheries Policy there is wide discretionary power for EU legislation, meaning that its decisions need not only be based on science. Furthermore no manifest errors had been made, a matter the Court could pass judgement onFootnote 8(Penca 2022).
Beam trawl fishery for sole
More than 95% of sole in the North Sea is landed by beam trawl vessels using a minimum cod-end mesh size of 80 mm. About 3% is landed by gillnetters operating in shallow coastal waters during the spawning period in spring. The beam trawl fishery is carried out in the area between 51 and 55°N (west of 5°E) or 56°N (east of 5°E) and is dominated by the Netherlands and Belgium with 75 and 7% of the annual landings, respectively (ICES 2023). The fishing vessels deploy a beam trawl on either side of the vessel. The width of each beam trawl is maximized at 4.5 m for small vessels (engine power ≤ 221 kW), that may fish in the coastal protection zone (12 nm zone, Plaice Box), and to 12 m for large vessels (engine power between 221 and 1491 kW) that may fish in offshore waters outside the coastal protection zone (Beare et al. 2013).
Conventional beam trawl (BT)
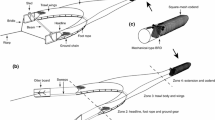
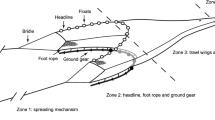
In BT an array of chains is attached to the shoes (shoe ticklers) or ground rope (net ticklers) to chase sole out of the sea bed (Fig. 2a). Depending on the type of sediment, tickler chains can disturb the sea bed to a depth of 8 cm (Paschen et al. 2000), although the mean penetration depth of a conventional beam trawl is estimated 2.72 cm (SD = 1.24) (Hiddink et al. 2017). To reduce the drag of the gear, most Dutch vessels replaced the iron beam and shoes by a hydrodynamic foil (Sumwing) that hovers above the sea bed lowering the fuel cost by 13% (Rijnsdorp et al. 2020a). In the Sumwing, the shoe ticklers are attached to the tips of the foil. The V-shaped ground rope is made of a heavy chain with rollers in the center (Rijnsdorp et al. 2021b).
Schematic drawing of a 12 m conventional tickler chain beam trawl BT (a) and a 12 m pulse wing trawl PT (b). For each gear the front (1) and bottom view (2) of the gear is shown with the rigging of the tickler chains from the shoes and ground rope (BT) or the rigging of the rectangular matrix of electrode arrays (vertical lines) and tension relief cords (dashed vertical lines) of the pulse trawl (PT). Bottom contacting gear components are the shoes, tickler chains and ground rope of the BT and the nose, ground rope and electrode arrays of the PT (adapted from Rijnsdorp et al. 2021b)
Pulse trawl (PT)
In a PT the tickler chains are replaced by a rectangular matrix of electrode arrays that are attached between the ground rope and the beam or the wing (Fig. 2b). Each electrode array comprises of a series of electrodes and insulated parts to reduce the power loss over the length of the electrode array (de Haan et al. 2016; Soetaert et al. 2019). Tension relief cords are deployed between the wing or beam and the ground rope to release the tension on the electrode arrays and maintain a rectangular shape. The electrode arrays run parallel in the towing direction at a distance of about 0.42 m of each other. PT is towed at a lower speed than BT (Table 1). The lower towing speed results in a fuel saving in large vessels of 33% when using a pulse-beam and 46% using a pulse-wing gear (Rijnsdorp et al. 2020a). More details of the pulse gears are given in (Depestele et al. 2019; Soetaert et al. 2019; Rijnsdorp et al. 2021b).
PT use a 30–45 Hz Pulsed Bipolar Current (PBC; alternating positive and negative pulses) with a pulse amplitude of about 56 V, a pulse width of 238–336 µs and a duty cycle of ~ 2% (Table 2).Footnote 9 The use of PBC increases the durability of the fishing gears because a Pulsed Direct Current (PDC) would corrode electrodes through electrolysis reactions (Tiano et al. 2021). The field strength is strongest (> 200 V m−1) close to the electrode and decreases exponentially with increasing distance from the electrode both in the water and in the sediment (de Haan et al. 2016; Boute 2022). Figure 3 shows the field strength around the outermost pair of electrode arrays in the horizontal and vertical plane (Boute et al. 2024). The black rectangle in Fig. 3b shows a part of the net opening on the right side of the net. The highest field strength in the net-opening occurs at the level of the sediment—water interface. The lowest field strength in the net-opening is 4 V m−1 at both sides of the net and between 5 and 10 V m−1 at the roof of the net. The field strength in the sediment is very similar to the field strength in the water column (de Haan and Burggraaf 2018; Boute 2022; Boute et al. 2024).
Maximum field strength (V m−1) around the outermost pair of electrodes on the right side of a pulse trawl. Left panel: horizontal plane at Z = 0 m with the electrode part in dark grey and the isolating parts in light grey. Right panel: vertical plane perpendicular to the electrode pair (black dots) at Y = 0. The black rectangle shows the position of the net opening at the right side of the net. The dashed line shows the sediment—water interface. Numbers denote lines of equal field strength (V m−1) (adapted from Boute 2022)
Changes in fishing effort during the pulse trawl period
The transition to PT took place in three waves following the successive issuance of pulse licenses between 2009 and 2016 (Haasnoot et al. 2016; Poos et al. 2020). During the pulse trawl period, the fishing effort of the pulse license holders (PLH), e.g. the subset of beam trawl vessels that made the transition from BT to PT, remained constant at around 13 thousand days when targeting sole in the sole fishing area (SFA), while the effort of non-license holders decreased between 2009 and 2014 from 7 to 3–4 thousand days (Fig. 4a). To compensate for the increased catch efficiency of sole (see Sect. "Catch efficiency and selectivity"), PLH increased their share of the Dutch sole quota from 73 to 93% by buying or leasing sole quota from other beam trawl vessels, while the proportion of the sole caught by PT increased to more than 90% of the Dutch sole landings (Fig. 4b). The proportion of plaice (Pleuronectes platessa) landed by PLH using PT decreased in line with their lower catch efficiency for plaice (Sect. "Catch efficiency and selectivity") but was compensated by an increase in effort north of the SFA targeting plaice (Fig. 4c). At the end of the pulse trawl period, effort and landings of PT declined as licenses were stepwise withdrawn in 2020 and 2021.
a fishing effort in 103 days at sea; b sole landings (103 ton); c plaice landings (103 ton) of the Dutch beam trawl fleet during the pulse trawl period from 2009 to 2021 showing the contribution of the pulse license holders (PLH) and other vessels (NO_PLH) using pulse trawls (PT) or conventional beam trawls (BT) fishing in the sole fishing area (SFA) or elsewhere (updated from Rijnsdorp et al. 2020b).
The spatial distribution of the PLH before and after the transition is broadly similar with most fishing activities occurring in the southern North Sea (Fig. 5). A closer look at the distribution maps shows a shift towards the southwestern part of the North Sea with an increase in fishing effort off the Belgium coast and in the Thames estuary. Beam trawlers exclusively trawled in soft sediments with a clear preference for sand and a negative preference for coarse and mixed sediments with only subtle differences between BT and PT (Rijnsdorp et al. 2020b; Hintzen et al. 2021). The similarity between BT and PT is at odds with anecdotal information from the fishing industry suggesting that PT moved into previously unfished muddy grounds in the southern North Sea (ICES 2018; Rijnsdorp et al. 2020a). This apparent contradiction may be due to the spatial resolution of the data that is too coarse to resolve the potential fine-scale variability in fishing effort related to the fine-scale pattern in sediment grain size and benthic community composition that is associated with the pattern of ridges and troughs within the 1 min latitude × 1 min longitude grid cells (van Dijk et al. 2012; van der Reijden et al. 2019).
Mean annual trawling intensity (SAR = swept area ratio per year of grid cells of 1 min latitude × 1 min longitude) of the Pulse License Holders using a conventional beam trawl before the transition (PLH_BT: 2009–2010) and using a pulse trawl after the transition (PLH_PT: 2016–2018). The horizontal line at 55°N and 56°N delineates the northern border of the sole fishing area (SFA) in the North Sea where a minimum cod-end mesh size of 80 mm is allowed (updated from Rijnsdorp et al. 2020b)
Catch efficiency and selectivity
Despite the lower towing speed, PT had a higher catch rate (kg h−1) of sole (Poos et al. 2020). A comparative study of the catch efficiency (kg ha−1) of the landing and the discard fractions of PT and BT showed that pulse stimulation changed the species selectivity where the catch efficiency of the species other than sole and whiting was reduced (Fig. 6). Due to the change in selectivity, the transition from the BT to PT resulted in a 36% decrease in discards (kg h−1) (95% prediction interval: 31–42%) (Van Overzee et al. 2023).
Differences in selectivity between large PT and BT trawlers as reflected in the catch multiplier (kg/ha) and 95% confidence interval of PT relative to BT for landings and discards (fraction of the catch below the minimum conservation reference size) of the target (sole) and main bycatch species (from van Overzee et al. 2023)
The change in species selectivity is likely related to the species-specific whole-body muscle contraction (denoted as cramp response in this paper). The cramp response, when the fish body is curved, will affect the ground-rope selectivity, e.g. the probability that a fish in the trawl track passes over the ground-rope and is retained in the cod-end (Rijnsdorp et al. 2021a; Van Overzee et al. 2023). The degree of body curvature differs between fish species and is strongest in sole as compared to plaice and dab (Supplementary Material SM1). The high catch efficiency of PT for sole may also be affected by the deeper penetration of the pulse stimulus into the sediment, which brings a larger proportion of sole buried in the sediment within reach of the gear (de Haan and Burggraaf 2018). No effect of pulse stimulation was observed on cod-end mesh selection (Rijnsdorp et al. 2021a).
The estimated reduction in discards concurs with the expected size-dependent response where large fish experience a stronger stimulus than small fish (Stewart 1975, 1977; Soetaert et al. 2015b, 2019). This interpretation is further supported by two comparative fishing experiments, one published (van Marlen et al. 2014) and one unpublished, where a PT fished side by side with a conventional BT. The comparative trawling experiments and the analysis of the discard monitoring data further showed that PT caught substantially less benthic invertebrates than BT (van Marlen et al. 2014; Van Overzee et al. 2023).
Effect of pulse exposure on marine organisms
Electric pulses affect organisms at the cellular, tissue and organismal level (Emery 1984). Pulses may activate neurons and may activate muscle fibers directly, or indirectly, through neuron activation (Soetaert et al. 2015b). A weak stimulus will result in the activation of a few neurons and/or muscle fibers (muscle twitch). Increasing the strength of the stimulus may lead to a muscle cramp or tetanus and may result in epileptic seizures and narcosis. Simultaneous contractions of the white muscle tissue on both sides of the vertebral column, that may coincide with epileptic events (Penfield and Jasper 1954), may cause a spinal injury and associated rupture of arteries or smaller blood vessels (Snyder 2003). Intense electrical stimulation may further result in cardiac or respiratory failure and immediate or delayed mortality (de Haan et al. 2016).
Sensitivity for electrical stimuli varies among fish species and is related to characteristics such as body shape, body size, anatomy and electrical properties of body components as well as to characteristics of the electric stimulus such as field strength, frequency, pulse shape and duty cycle (Sternin et al. 1976; Snyder 2003a; Soetaert et al. 2015b; Beaumont 2016). Peak-voltage-gradient threshold data for a minimum (muscle twitch) and maximum response (tetanus, stunning or narcosis) for a variety of fish species, exposed in the laboratory to a direct current (DC), alternating current (AC) or pulsed direct current (PDC), have been reviewed by Sternin et al. (1976) and Snyder (2003a). These and more recent studies are compiled in Table SM2.1 of the Supplementary material. Although no standardized methodology was used across the different experiments, the results provide an indication of the approximate response thresholds. Thresholds decreased with increasing water conductivity and were generally lower for AC than other waveform types (Fig. 7). The minimum response threshold for PDC in brackish and sea water (> 10 mS cm−1) ranged between 0.07 and 0.14 V cm−1 and was close to the behavioural response thresholds estimated for the PBC stimulus used in the pulse fishery for sole (Boute et al. 2024): sole 0.06 V cm−1, turbot (Scophthalmus maximus) 0.08 V cm−1, European seabass (Dicentrarchus labrax) 0.06 V cm−1, small-spotted catshark (Scyliorhinus canicula) 0.10 V cm−1, thornback ray (Raja clavata) 0.08 V cm−1 (Fig. 7a). The cramp response threshold of cod exposed to a PBC stimulus (0.37 V cm−1, de Haan et al. 2016) also fell within the range of PDC threshold values reported in the literature (Fig. 7b).
Response thresholds (V cm−1) for a minimum (muscle twitch) response (a) and maximum (stunning, narcosis, tetanus) response (b) of various fish species exposed to an alternating current (AC), direct current (DC), pulsed direct current (PDC) and a pulsed bipolar current used in the pulse trawl fishery for sole (PBC) for different conductivities (mS cm−1). Data from Table SM2 of the Supplementary Material
Laboratory experiments
The experiments carried out to study possible adverse effects of the pulse stimuli used in the fishery for sole are summarized in Table 3. These laboratory studies focused on a range of organisms (from benthos to (non-) electroceptive fishes; from eggs to adults) as well as electrical parameters. Experiments were conducted with either plate-shaped electrodes, creating a homogeneous electric field, or wire-shaped electrodes creating a heterogeneous electric field. Experiments with a homogeneous electric field give much better control over the field strength the organism is exposed to. A heterogeneous electric field is more similar to the in situ field of a commercial PT, but has the disadvantage that the exposure strength is less well defined. In a heterogeneous field, the local field strength around the circumference of a fish varies with distance and angle to the electrode pair, whereas in a homogeneous electric field the local field strength is similar along the circumference of a fish assuming the electrical conductivity of the fish and the ambient conductivity of the water are the same. In reality, the electric field is influenced by the presence of an organism due to the differences in conductivity between the water or sediment and the fish (Sternin et al. 1976; Soetaert et al. 2019). In experiments with a heterogeneous electric field, the maximum local field strength along the circumference of the fish was generally used as a metric of the field strength to which the animal is exposed. Animals were generally monitored for a few days to up to 9 months to record mortality and study the recovery after exposure before animals were euthanized for autopsy and examination of pulse-induced injuries. Some experiments exposed animals to a pulse stimulus that was stronger, or of longer duration, than animals would experience in situ.
Fishes
Low frequency pulses (5 Hz) induced a muscle contraction at every pulse and elicit a flight response (Desender et al. 2016; Soetaert et al. 2016b). Increasing the pulse frequency to 20 Hz or more elicited a cramp response that immobilized the fish as long as the duration of the pulse (Soetaert et al. 2016b, 2019). After cessation of the pulse stimulus, fish often showed a strong swimming response (de Haan et al. 2015; Soetaert et al. 2016b). When the field strength or pulse duration was raised, a stronger response could be elicited, such as an epileptic seizure or sedation. In cod, epileptic seizures were observed immediately after cessation of the pulse stimulus, during which the fish showed a lack of responsiveness for a short period of time (minutes) (de Haan et al. 2016; Soetaert et al. 2016a, 2016b).
Pulse-induced injuries and mortality were mainly observed in marketable sized (> 35 cm) cod (de Haan et al. 2008, 2011, 2016). In the experiment where cod were exposed for 4 × 1 s in a heterogeneous electric field, three out of 20 cod exposed to 82 V m−1 died immediately after exposure, one cod died within one day, and two died within the two week observation period after exposure, whereas none of the 40 cod exposed to a field strength of 4 or 37 V m−1 died (de Haan et al. 2011). Mortality within two days after exposure was also observed in 13 out of 260 cod > 35 cm exposed for 1 s to a pulse stimulus between 37 and 103 V m−1, while no mortality was observed among 140 small cod (< 20 cm) exposed to a field strength of 76–370 V m−1 (de Haan et al. 2011). Exposure to a strong pulse stimulus also affected the appetite of fish. Cod (> 35 cm) exposed to 82 V m−1 were passive and did not resume normal feeding at the first offering of food 36 h after exposure. The appetite of fish exposed at 37 V m−1 increased during the observation period and was higher than the appetite of fish exposed at 4 V m−1 and “control” fish. Small cod (< 20 cm) exposed to a field strength between 76 and 370 V m−1, some of which showed an epileptic seizure, resumed normal behaviour and responded to food within a few minutes (de Haan et al. 2016).
X-ray analysis and autopsy revealed that 39–45% of the 35–60 cm cod showed either a vertebral fracture, a haemorrhage, or both, in contrast to small cod (11–17 cm) of which none were injured (de Haan et al. 2008, 2011, 2016). An internal injury often coincided with an external discoloration mark. Injury probability was related to the field strength with an inflection point of 85 V m−1 (de Haan et al. 2016). The sensitivity for pulse-induced injuries appeared to be size-dependent with the highest sensitivity for intermediate sized cod (35–40 cm).
Lower injury rates (2.5%) were observed in the experiment of Soetaert et al. (2016b) who exposed cod for 2 s to square shaped pulses at a field strength of 100, 150 or 200 V m−1 in a homogeneous electric field. All cod survived during the 14 days observation period. With the exception of a spinal column dislocation in one cod, no lesions attributable to electric pulses were detected by gross and histological examination. Also no signs of hypoxic damage that might have been caused by possible cardiac arrest during exposure, or respiration arrest during epileptiform seizures, were observed.
To further investigate the difference in pulse-induced injury rates observed between the experiments of de Haan et al. (2016) and Soetaert et al. (2016b), a third experiment was conducted. Of the 170 cod exposed close to electrode three cod (1.1%) developed a haemorrhage and spinal fracture and two (1.7%) developed a haemorrhage. No significant difference was found in the number of injuries or haemorrhages between wild and farmed fish and different broodstocks or generations of offspring exposed. Necropsy of animals without spinal injuries revealed no macroscopic external or internal acute lesions. The experiment confirmed that cod showed a variable sensitivity suggesting that injuries are not only determined by the pulse parameter settings and body size but also by unknown fish-specific factors (Soetaert et al. (2016a)).
In contrast to cod, flatfish appeared insensitive for pulse-induced injuries. Soetaert et al. (2016b) exposed sole to electric pulses using the same parameters applied to cod. All sole showed a cramp response but resumed normal behaviour after exposure. Over the 14 day observation period, a single sole succumbed on day 13, but gross and histological examination of this fish and other exposed individuals, did not reveal lesions attributable to electric pulses. Dab (Limanda limanda) exposed to a pulse stimulus of 240 V m−1 for 1 s responded well to the food offered daily during a 5-day observation period (de Haan et al. 2015). In this experiment, two of the 102 exposed fishes died after the treatment without a clear relation to the treatment. Gross, histological and microbiological analysis showed no significant differences in external and internal anomalies in the exposed and control group.
European seabass, exposed for 2 s to a pulse stimulus at field strengths between 37 and 155 V m−1, showed a cramp response during exposure and survived during the 2-week observation period (Soetaert et al. 2018). After exposure the fish showed normal feeding behavior and no injuries were found during autopsy, using gross, radiographic and histologic examination.
Sandeels were exposed to a pulse stimulus for 2 s in an experimental cage with 5 cm sediment (Schram et al. 2022b). The field strength varied between 20 and 640 V m–1, depending on their positions relative to the electrodes. X-radiography and dissection revealed that none of the sandeels showed a spinal injury or haemorrhage. In the absence of spinal injuries, the field strength below which spinal injuries probability ≤ 1% was estimated by bootstrapping at 320–540 V m−1.
Elasmobranchs are known to be extremely sensitive for low-frequency pulses (Dijkgraaf and Kalmijn 1963; Boute et al. 2024). To address the concern about possible adverse effects of pulse trawling, three groups of 16 small-spotted catshark (Scyliorhinus canicula) were exposed to high frequency pulses with a field strength of 8, 48–62 or 162 V m−1 (de Haan et al. 2009). All fish resumed feeding and did not show any injuries. During 7 months post exposure the three exposed groups produced between 5 and 39 egg capsules per group in contrast to the control group that did not. Two fish died 8 and 9 months after exposure but their death could not be linked to the pulse stimulus. To study whether pulse stimulation may disrupt prey detection using their electro-sense organs, small spotted catshark were trained to bite into an artificially created prey-simulating electrical field that was hidden in the sediment. No significant difference was observed in the detection ability of the catsharks exposed to a high-frequency sole pulse or shrimp pulse and the control fish (Desender et al. 2017b).
In conclusion, the available experiments showed that pulse stimulation resulted in a cramp response in all species tested and inflicted internal injuries and mortality in cod. The sensitivity of cod varied with body size with highest sensitivity for intermediate size groups. The five other species tested, including an electro-receptive species, appeared to be insensitive.
Benthic invertebrates
In an exploratory study a limited number of specimens from different taxonomic groups (echinoderms, crustaceans, bivalves, polychaetes) were exposed to a commercial pulse stimulus with a pulse amplitude that was twice as high and a duration eight times as high as in the fishing practice (Smaal and Brummelhuis 2005). None of the groups showed a difference in survival between the exposed and control group during three weeks following the exposure. In a follow-up study, specimens of six benthic invertebrate species were exposed to a commercial sole pulse at three levels of field strength (van Marlen et al. 2009). A Kaplan–Meier re-analysis of the data did not show a difference in mortality between exposed and control durig the two week observation period after exposure.
Brown shrimp (Crangon crangon) and king ragworm (Alita virens) were exposed to a commercial sole pulse at different field strengths (Soetaert et al. 2015a). No significant increase in mortality or injuries was observed. Examination of the hepatopancreas of shrimp exposed to 200 V m−1 showed a significantly higher severity of an intranuclear baculoform virus (IBV) infection. In a follow up study, shrimps were exposed to a series of 20 pulses in 4 d (Soetaert et al. 2016c). Survival of shrimps exposed to the sole pulse (57%) was significantly lower than in the control group (70%). In contrast to the previous study, no effect on the severity of IBV infection was found.
Exposure of six benthos species to a commercial sole pulse showed a variable response between no visible effect (echinoderms) to squirming (sea mouse) and retractions (whelk and crustaceans) (Boute et al. 2021) Within 30 s after stimulation, all animals resumed normal behavioural patterns, without signs of lasting immobilisation. For none of the species, survival at 14-days after stimulation was reduced. About two-thirds of the whelks ejected a white substance during or immediately after stimulation, presumably related to reproduction.
The impact of a sole pulse on the functioning of invertebrates was studied by Tiano (in Rijnsdorp et al. 2020a). Lugworms (Arenicola marina), exposed in their burrows, exhibited muscle contractions but resumed burrowing and bio-irrigation activity within 5–10 min after exposure. Due to the temporary halt in burrow ventilation a decrease in the sediment oxygen was observed which increased to prior levels as bio-irrigation resumed. No pulse-induced mortality was observed. In a separate experiment involving ocean quahogs (Arctica islandica), individuals with initially open valves promptly closed them upon exposure to electrical stimuli. Some individuals remained closed for several days, however, other individuals opened their valves within minutes after exposure. No mortalities were recorded within 12 months.
In conclusion, the available studies showed that the exposure to a pulse stimulus at a field strength of 150 or 200 V m−1 did not increase the mortality rate in 13 out of 14 benthic invertebrate species, and most of the species quickly resumed their normal behaviour after exposure. Results for brown shrimps were ambiguous. Brown shrimps exposed to a single stimulus did not show an increased mortality, but when exposed to a very intense pulse stimulation mortality was increased.
Bio-geochemical processes
The impact of pulse stimulation on benthic biogeochemistry was investigated by exposing sediment core samples from the North Sea seafloor to a homogeneous electrical field which encompassed the entire sediment core and overlying water (Tiano et al. 2021). Following 3-s exposure treatments, no discernible effects on oxygen or nutrient fluxes were observed (Fig. 8). In contrast, mechanical disturbance reduced bottom water oxygen levels and released nutrients from the sediment porewater. Electrical-induced impacts on biogeochemistry were only observed when high frequency pulsed direct currents (PDC) were applied to sediments for a duration of 2 min. These samples featured declining pH and phosphorus in the bottom water caused by the unidirectional movement of electrons. However, these electrolysis-induced effects would not be possible in the PT which employed a PBC, with sediments being exposed for approximately 1.5 s. Even with a two-minute exposure treatment, samples exposed to PBC in this experiment showed no biogeochemical effects (Tiano et al. 2021).
Electrical and mechanical-induced changes for oxygen saturation and ammonium, phosphate and silicate concentrations between experimental treatments in the water column of sediment cores. *p < 0.05, **p < 0.01, ***p < 0.001 significant differences compared to control samples (from Tiano et al. 2021)
Field studies
Pulse-induced injuries
The occurrence of pulse-induced injuries was studied by sampling fish on board commercial pulse trawlers (Boute et al. 2022, 2023). Pulse-induced injuries were diagnosed when fish showed both a spinal injury and a haemorrhage at the same location, similar to the injuries observed in laboratory experiments (Snyder 2003a; de Haan et al. 2016; Soetaert et al. 2016b). Pulse-induced injuries were observed in cod, whiting (Merlangius merlangus), grey gurnard (Eutrigla gurnardus) and greater sandeel (Hyperoplus lanceolatus), but the rate of occurrence was low (< 2%) in all species studied except cod (30%) (Table 4). Injury rate in cod was size-dependent with highest rates in cod of 25–30 cm, corroborating the results of the exposure experiments (de Haan et al. 2016). No pulse-induced injuries were observed in flatfish (sole, plaice, dab) and dragonet (Callionymus lyra), bib (Trisopterus luscus) and lesser weever (Echiichthys vipera). The diagnosis of pulse-induced injuries was validated by comparing injuries in ten species sampled from PT and BT trawlers, as well as in four species sampled from PT with the stimulus switched on or off. Because laboratory experiments (de Haan et al. 2016; Soetaert et al. 2016a) showed that some of the exposed cod showed a major haemorrhage in absence of a spinal fracture, the diagnostic is a conservative estimate of the pulse-induced injury rate. The total injury rate including fish with either a spinal injury or a major haemorrhage, which include both pulse-induced and mechanically-induced injuries, ranged between < 1% and 40% in PT and between < 1% and 42% in BT (Table 4, Fig. 9).
Probability and 95% confidence interval of major spinal injury or major haemorrhage (s2|h2) in fish species caught by pulse trawls (PT) with the pulse stimulus switched on (green) and off (orange) and by conventional tickler chain beam trawls (BT). Species codes: SOL—sole; DAB—dab; PLE—plaice; COD—cod; BIB—bib; WHG—whiting; GUG—grey gurnard; GUU—tub gurnard; YEZ—greater sandeel; ABZ—lesser sandeel; LYY—common dragonet; BSS—sea bass; TOZ—lesser weever (from Boute et al. 2023)
Discard survival
The effect of pulse exposure can also be inferred by comparing the discard survival rate of fish sampled on board of commercial PT and BT vessels (Table 5). Survival rates of PT flatfish discards ranged between 13–30% and were substantially higher than the survival rates of 3–6% reported for BT discards. Survival rates for rays were higher (45–53%) but did not differ between both gears.
Although the very low discard survival of plaice and sole in the Dutch BT fishery in the 1970s and 1980s are not necessarily representative for the present day survival due to differences in methodology and fishing practice (van der Reijden et al. 2017), discard survival was equally low in two recent trips of large Belgian beam trawl vessels (Uhlmann et al. 2021). A lower survival of BT discards is also suggested by the lower vitality scores that are predictive for the survival probability (Uhlmann et al. 2016; Schram et al. 2020). In four flatfish and two species of rays, the percentage of fish that were in poor condition (vitality score C or D) ranged between 53 and 92% in the BT discards and between 25 and 65% in PT discards (Schram et al. 2020).
The occurrence of external injuries is substantially higher than the occurrence of internal pulse-induced injuries suggesting that injuries are predominantly due to mechanical damage inflicted during the catch process (high towing speed, hard and sharp objects in catch (shells, stones, sand, etc.), and will be reduced if the tickler chains are replaced by electrical stimulation.
Trawl-track mortality
To investigate the claim of small-scale fishers and environmental NGOs that pulse trawling would create a ‘graveyard’ in the wake of the PT (Bloom 2018), a field experiment was designed in collaboration with representatives of the small-scale fishing industry (Schram et al. 2022a). Using a small-meshed shrimp trawl, samples were taken in the trawl track of a PT and just outside the trawl track to estimate the direct mortality among three dominant fish species and four dominant invertebrate species. Direct mortality among fish and invertebrates was low (0–10%) and did not differ between the PT track and the untrawled controls. Equally, no impact of pulse trawling was found on external damages and vitality scores. The experiment refuted the claim that PT causes mass mortality among marine organisms in the trawl track.
Impact on benthos, sea floor habitats and bio-geochemistry
In an attempt to study possible long term effects of pulse trawling, (Ford et al. 2019) compared the abundance and composition of the fish and benthic community between an offshore and inshore area on either side of the border of the 12 nm coastal protection zone. The offshore area was intensively trawled by PT vessels and the inshore area was an important fishing ground for smaller vessels using static and towed gears. Although the species richness and abundance was generally lower in the offshore area, the difference could not be ascribed to pulse trawling because the observed difference could also be due to the long-term differences in trawling intensity, as no un-trawled reference sites for inshore and offshore area were available.
Field experiments which quantified the direct impact of the passage of a commercial BT and PT gear on sea floor habitats and benthos in areas that are representative for the fishing grounds of the beam trawl fleet are reviewed below. The impact reflects the effect of mechanical disturbance and, in the case of PT, mechanical and electrical disturbance.
The physical impact of a commercial 4 m BT and a PT in a sandy sediment area of the shallow coastal-zone of the southern North Sea was assessed by Depestele et al. (2016). The study showed that the seabed bathymetry changed between 1 and 2 cm and that the alteration following the passage of a BT was greater than that following the passage of a PT. There was no difference in the quantity of sediment mobilized in the wake of the two gears. In a follow-up study the impact of a commercial 12 m BT and PT was studied offshore in fine muddy sand (Depestele et al. 2019). The BT consistently and uniformly deepened the tracks to 1.5 cm depth in contrast to 0.7 cm following PT trawling. BT trawls flattened seabed roughness significantly more than PT. Sediment Profile Imagery (SPI) showed that BT homogenized the sediment deeper and removed more of the oxidized layer than PT. Total penetration depth of BT (mean = 4.1 cm, SD = 0.9 cm) was larger than PT (mean = 1.8 cm, SD = 0.8 cm).
The impact of a conventional BT with two alternative beam trawl gears (PT with longitudinal electrodes, BT with longitudinal chains) on the mortality in benthic megafauna was studied by Bergman and Meesters (2020). The PT used in the field experiment was the prototype 7-m pulse trawl of the late nineties (Van Marlen et al. 2001). The study showed higher mortalities in a majority of species after longitudinal than after conventional BT and lower mortalities after PT. Although PT can reduce the impact, the average mortality remains substantial (25%) and the statistical power was generally low.
A study of the acute impact of a BT and PT in the Frisian Front area of the North Sea showed that bottom trawl disturbance can lead to immediate declines in benthic community metabolism, with BT exhibiting more prominent alterations than PT on benthic biogeochemical processes (Tiano et al. 2019). A reduction of sedimentary chlorophyll a was observed, which was larger following BT (83%) compared to PT (43%). This displacement of surface material caused significant decreases in the sediment oxygen consumption in BT (41%) and PT samples (33%) along with a deeper penetration of oxygen in the sediment (BT: 3.78 mm, PT: 3.17 mm) compared to untrawled areas (2.27 mm). Both BT and PT flattened and homogenized the surface sediments. Both trawl gears induced significant changes to infaunal communities, with no differential effect between the two gears (Tiano et al. 2020).
The physical, biological and biogeochemical effects of BT and PT was studied in a coastal ecosystem dominated by the tube building polychaete, Lanice conchilega (Tiano et al. 2022). With a before-after-control-impact (BACI) in situ study, a ~ 1 cm bathymetric deepening after trawling was detected associated with significant losses in benthic chlorophyll a caused from both fishing gears. BT significantly reduced sediment oxygen consumption (57%), total organic matter mineralization (56%), denitrification (61%), nitrification (60%), and total benthos densities (52%) compared to PT which displayed no statistically significant impact on these parameters. Before trawling, significant relationships were found between L. conchilega and very fine sand fractions, oxygen and nitrate fluxes, taxon densities and species richness, however, the trawl disturbances from both gears disrupted these connections. The results suggest a reduced mean effect for PT compared to BT for several ecological and biogeochemical characteristics though their impact was still significant for L. conchilega and associated species.
In conclusion, the field experiments showed that sea floor disturbance (flattening of the sea floor, sediment mixing, penetration of gear components in the seafloor) and impact on the benthic ecosystem (benthos mortality) and biogeochemical process, was generally smaller for PT than for BT.
Assessing the impact on the population level
The population level impact of electrical stimulation depends on the sensitivity of the species, e.g. field strength threshold above which adverse effects occur, and the proportion of the population exposed above the threshold. In the following sections we integrate information on the field strength thresholds above which pulse stimulation may inflict damage with the pattern of field strengths in the mouth of a PT (Fig. 3) and observations on the annual trawling frequency (Fig. 5). Here we present the results of an updated analysis of which the details are presented in Supplementary Material SM3. Results of an earlier analysis are included in the final report of the IAPF project and the ICES expert group report (ICES 2020a, Rijnsdorp et al. 2020a).
Impact on a theoretical population
At the height of the PT period, 6% of a theoretical population inhabiting the fishing grounds of the PT fleet would have been exposed 1 year−1 to a pulse stimulus above 200 V m−1, the threshold where exposure experiments did not find any adverse effects (Sect. "Effect of pulse exposure on marine organisms"), and < 1% would have been exposed 2 year−1 (Figure SM3.2). This estimate applies to animals that pass through the strongest electric field less than 5 cm above or below the array of electrodes.
An analysis of repetitive exposures in the three most intensively trawled ICES rectangles of 0.5° latitude and 1° longitude showed that less than 0.3% of the pixels at the size of the trawl were exposed to a pulse stimulus > 4 V m−1 for a second time within a week and less than 0.16% when the time interval was reduced to 1 day (ICES 2018). The low probability of repetitive exposures is consistent with the tactics of pulse trawl fishers who were shown to trawl a local fishing ground by putting trawl tracks parallel to a previous track at a median distance of 200 m and avoid trawling the same area again during a fishing trip (Rijnsdorp et al. 2022).
We conclude that the PT fleet does not impose a chronic exposure of marine organisms as the exposure probability to a field strength above which adverse effects could occur is low and the risk of multiple exposures over a period of days can be considered to be negligible.
Impact on cod
Among the species studied, cod is shown to be the most sensitive species for pulse exposure. To assess the potential population level impact we focus on the fate of small cod that are exposed to a pulse stimulus but escape through the meshes. Assuming that all of the small cod that enter the net but escape through the cod-end meshes will die when exposed to a field strength above the threshold for pulse-induced injuries (85 Vm−1, de Haan et al. 2016), PT would reduce the equilibrium spawning stock biomass by 1.6% (Supplementary Material SM3.3). Because exposure experiments indicated that the sensitivity for pulse-induced injuries was lower in small cod (de Haan et al. 2016) and only part of the southern cod stock occurs in the fishing area of the PT, we consider the impact of pulse trawling on the cod population to be negligible.
Impact on sole
Concerns were raised by stakeholders about possible non-lethal effects on reproduction of marine species (Kraan et al. 2015). Since sole is the target of the PT, this species is expected to have the highest exposure frequency to pulse stimuli. Non-lethal effects could occur if sole are exposed to a pulse stimulus but escape through the cod-end mesh. Supplementary Material SM3.4 shows that 65% of the population of adult soles, that survived to a size at which they can no longer escape through the cod-end meshes (30 cm and 4.5 years), have never encountered a pulse stimulus of > 5 V m−1, 27% encountered a pulse stimulus once, 7% encountered a pulse stimulus twice and 1% encountered a pulse stimulus 3 or 4 times. It was further shown that 83% of the maturing soles were not exposed during the year before first spawning, 16% were exposed once and 2% were exposed 2 or more times during their maturation.
Although no studies have been conducted on the possible adverse effects of non-lethal exposure on the maturation process, the quality of gametes, or spawning behaviour of any fish species, the short duration of the pulse stimulus (1.5 s) and the low exposure probability to a field strength of > 5 V m−1, well below the maximum field strength tested where exposure experiments did not show harmful effects, makes it highly unlikely that pulse trawling will impair the reproductive capacity of the sole stock. This inference is supported by the year class strength of 0- and 1-group sole observed in the annual beam trawl surveys that does not show a reduced year class strength during the pulse trawl period. The 2018 year class, born at the peak of pulse trawl effort, was one of the largest since the start of the fisheries-independent surveys for this stock in 1985 (ICES 2023).
Impact on eggs and larvae
No studies are available on the impact of the sole pulse on early life stages, but two studies exposed early life stages of cod and sole for 5 s to a square-wave PDC of 150 V m−1 and a lower frequency (5 Hz) with ambiguous results (Desender et al. 2017a, 2018). In cod, an increased mortality was reported in two out of eight egg and larval stages tested, whereas no effect was found in sole. No deviations in yolk sac resorption and morphometric length measurements of the notochord, muscle, eye, and head were observed in both species.
Although these results do not exclude a possible pulse-induced effect, the probability that pelagic eggs will be exposed to a pulse stimulus of > = 150 V m−1 will be very low because of their pelagic distribution in the water column and the short stage duration (weeks). A study of the overlap in distribution between the egg and larval stages of sole and the pulse fleet indeed showed that only 0.02% of the eggs were exposed to a pulse stimulus (Rijnsdorp et al. 2020a). Even if all of these eggs would die, the pulse-induced mortality rate would be insignificant given the daily mortality rate in sole eggs of 30% or more (review in Horwood 1993). Although species with demersal eggs, such as sandeel, may be exposed more frequently, the estimated cumulative mortality imposed by pulse exposure (0.4%) was low (Rijnsdorp et al. 2020a) compared to the estimated daily mortality of 7–50% (Bunn et al. 2000). The low exposure rates during the egg stage and the fact that many fish populations are regulated by density-dependent processes after the egg stage (Leggett and Deblois 1994; Lowerre-Barbieri et al. 2017) we consider it highly unlikely that pulse exposure will have a negative effect on the reproductive success of a population.
Impact on benthos and sea floor habitats
Because there is no evidence for adverse effects of electrical stimulation the impact of PT on the sea floor will be due to mechanical disturbance. Given the lower towing speed and higher catch efficiency for sole, the transition from BT to PT reduced the surface area of the sea floor swept annually by PLH between 2009 and 2015 by 26% (Fig. 10a). The impact on the benthic ecosystem will be reduced further because of the lower penetration depth of PT into the sediment (Depestele et al. 2016, 2019) and the concomitant lower mortality imposed among benthos (Hiddink et al. 2017; Sciberras et al. 2018). The impact of PLH on the relative benthic status (RBS sensu Pitcher et al. 2017) was estimated to be reduced by 60% from 0.026 in 2009 to 0.011 in 2015 at the height of pulse trawling (Fig. 10b). Taking account of the increase in the proportion of sole landed by PLH (0.95/0.7), the reduction in impact per unit of sole quota (80%) was even higher. At the end of the pulse trawl period, the impact increased to 0.021 in 2021 when most PLH had switched back to BT. Applying two other methods, a smaller reduction in impact was estimated (20 and 39%; Rijnsdorp et al. 2020b). These latter methods, however, are less suitable as they are only sensitive over a narrow range of rather low trawling intensities (Rijnsdorp et al. 2020c).
a Change in the area swept annualy and b Change in benthic impact expressed as the reduction in relative benthic status (1-RBS) in the sole fishing area. Red line: PLH using PT; blue line: PLH using PT or BT; black line: total Dutch beam trawl fleet (update of Rijnsdorp et al. 2020b).
The transition from BT to PT also reduced the amount of sediment mobilized, in particular for large vessels, due to the lower hydrodynamic drag (Rijnsdorp et al. 2021b). The consequences of the reduced sediment disturbance on the bio-geochemistry was studied by simulating the effects of BT and PT in five different habitats with a biogeochemical model (OMEXDIA; Soetaert et al. 1996). Due to the disturbance of the biogeochemically active surface sediment layer, the long-term impact of both types of gears led to significantly less ammonium and organic carbon while reducing carbon degradation and sedimentary CO2 release. PT had a slightly lesser effect on denitrification compared to BT, however, no significant differences between the two gear types were observed for all other biogeochemical parameters (De Borger et al. 2021).
The food web consequences of the transition to PT was explored using a modelling approach that allowed for a simultaneous change in direct, lethal effect and indirect, non-lethal effects of trawling (van de Wolfshaar et al. 2020a). The model showed that although benthic invertebrates may respond to a pulse exposure by slowing down their normal activities, the duration of this effect is short and is unlikely to affect the macro-invertebrate food web (van de Wolfshaar et al. 2020b).
The above results provide strong support that a transition to PT reduces the adverse impact on benthos and sea floor habitats.
Socio-economic consequences
The transition to PT greatly improved the economic profits of the beam trawl fleet (Turenhout et al. 2016). The transition remained limited to fishing companies owned by Dutch fishers, including foreign flagged companies in the United Kingdom, Germany and Belgium (Hamon et al. 2016). The main driver that influenced the technological change was the good economic performance of the pulse gear (Fig. 11). The investment in PT was often quickly earned back by fishers with a significant sole quota. This also explains why it was mainly Dutch fishers that profited from the innovation, as (pre-Brexit) the Netherlands held 74% of the EU North Sea sole quota. But also non-financial factors played a role. In particular, the information sharing amongst fishers through the group of 5 pioneers and demonstration days accelerated the process. Fishers also valued the cleaner catches (less debris and bycatch) which resulted in less time spent to sort the catch and better quality fish. The support of the Dutch government was also influential. In contrast, barriers such as limited days at sea in the North Sea for Belgian fishers and the controversial image of the PT in Belgium have hindered its adoption (Hamon et al. 2016).
Net result (106 Euro) of the Dutch beam trawl fleet using the conventional beam trawl (BT) or pulse trawl (PT). Data from https://www.wur.nl/nl/onderzoek-resultaten/onderzoeksinstituten/economic-research/show-wecr/visserij-in-cijfers-2023.htm
The transition to PT had profound implications for the interactions with other fishers. The improved selectivity for sole, and the possibility to deploy the lighter PT on fishing grounds that were previously inaccessible to the conventional BT, increased competition with other vessels fishing on the same fishing grounds. When Dutch beam trawl vessels switched to PT, parts of the Belgian beam-trawl fleet reduced their fishing effort in the southern North Sea (Vansteenbrugge et al. 2020). This response is likely due to the increased competition with PT since those large Belgium beam trawlers, that continued fishing in the southwestern North Sea, experienced a decrease in catch rate during weekdays when the PT vessels were present, but not during the weekends when the PT vessels were in port (Sys et al. 2016). Also small scale fishers from several North Sea countries, including the Netherlands, united in the organisation LIFE, voiced their concern about competition with PT which they claimed caused the falling catch rates (Anon 2017; Steins 2018; Ford et al. 2019). A desk study concluded that the decline in the gillnet catch of sole, but not of cod and seabass, was likely due to the competition with pulse trawlers (Rijnsdorp et al. 2018b).
There are two mechanisms that can explain the increased competition between PT vessels and other vessels on local grounds. The first is the higher catch efficiency of PT that will increase the local depletion rate making the local ground less attractive for other vessels. The second mechanism is related to the response of fish to the fishing activities which make fish more difficult to catch (Gillis 2003). In a comparative study of the catch rate on local fishing grounds of BT and PT vessels, it was shown that the catch rate per tow of PT declined at a slower rate than of BT despite the higher catch efficiency of PT, suggesting that PT activities result in a weaker avoidance response than BT (Rijnsdorp et al. 2022). This may also explain the complaint of coastal gill netters that their catch rate dropped when PT vessels trawled in close proximity but not when BT vessels trawled in close proximity when they were fishing for sole during their onshore spawning migration. This tentatively suggests that BT trawling, although reducing the local abundance of sole, may positively affect the catch rate of gill netters by chasing sole into the gillnets.
The ban on pulse fishing in 2021 had profound consequences for the pulse fishers, as they had to revert back to beam trawling. Revenue was estimated to become negative with an annual loss of 100.000–120.000 Euro per vessel (Zaalmink et al. 2018). The economic data for 2022 confirmed that the beam trawl fishery was making losses (Fig. 11). The poor economic results were aggravated by the increasing cost of fuel and the loss of fishing opportunities due to Brexit.Footnote 10
Discussion
The research conducted during the ‘gear transition period’ provided compelling evidence that the application of electrical stimulation in the beam trawl fishery for sole can improve the ecologic and economic sustainability of the fishery. The expansion of licenses beyond the 5%-derogation in 2011 and 2014 was agreed by the EU Commission with differing conditions for research. Although the large number of pulse licenses was not required to study research questions that were formulated by ICES and the STECF at the start of the study period, it created a unique opportunity to study the consequences of a gear transition at the level of the fleet by comparing the impact of the PLH when using a BT and PT before and after the transition, respectively.
The transition to PT improved the economic profitability of the beam trawl fishery for sole because of an increase in the catch efficiency for the main target species (sole) and lower fuel cost (Turenhout et al. 2016). The reduced fuel consumption also contributes to the goal of the Paris agreement on climate change to reduce the emission of greenhouse gasses.
The ecological sustainability benefits from the improved selectivity (more sole, less plaice and other flatfish), which resulted in a decrease in bycatch of fish and benthos, and the reduction in the area swept and disturbance of the sea floor and benthic ecosystem. The results refute the claims by environmental NGOs and mainly small-scale fishing interest groups that pulse trawling leads to the electrocution of marine life and turn fishing grounds into a graveyard (Le Manach et al. 2019; Schram et al. 2022a).
The adverse effects of electrical stimulation studied in 13 fish species, representing almost 90% of the discard numbers, revealed pulse-induced injuries in four species. The injury probability was generally low (< 2%) (Boute et al. 2023) and much lower than the proportion of fish that are injured by mechanical forces during the catch process (% discards in poor condition: PT 25–65%; BT 53–92%; Fig. 4 in Schram et al. (2020)). Of the species studied, only cod was found to be sensitive for pulse-induced injuries. Because of the relative small proportion of the cod population that is exposed to a pulse stimulus and the lower injury probability of cod size classes that can escape through the cod-end meshes, the population level effects are considered to be negligible. As we lack a mechanistic understanding why cod is so much more sensitive for pulse-induced injuries than other fish species, additional research is required to study the sensitivity of fish species occurring in other management areas to assess the impact on the sustainability of PT in other management areas.
Although there are still many unknowns about the physiological effects of pulse stimuli on non-commercial marine species, it is unlikely that there will be serious ecological consequences. The reason is that the probability that organisms will be exposed to a pulse stimulus exceeding the maximum field strength used in the exposure experiments where no damage was inflicted (200 V m−1) is small and the pulse stimulus has only a very short duration (~ 1.5 s) and a low duty cycle (2%). Also the risk of chronic exposure will be negligible as the chance that an organism will be exposed multiple times is very low. Adverse effects on eggs, larvae and planktonic organisms are equally unlikely because of the low exposure probability and the high rate of natural mortality of these early life stages.
The transition to PT will have implications for animal welfare. Exposure to a pulse stimulus and the associated cramp response and possible internal injuries will certainly cause discomfort. On the other hand, there are factors that likely reduce discomfort, such as the lower towing speed and absence of tickler chains. BT catches a larger volume of hard sea floor objects (stones, shells, sand) that may damage fish during the tow (Veldhuizen et al. 2018). Because fish caught in BT show a higher incidence rate of internal and external injuries, we speculate that PT will reduce the overall discomfort during the catch process. The lower catch volume in PT will further reduce the discomfort during the shorter duration of the processing of the fish on deck.
The increased catch efficiency of the pulse trawl could in theory jeopardize the sustainable exploitation of the sole stock. North Sea sole is managed under the EU Common Fisheries Policy (CFP) by a total allowable catch (TAC) set to maintain the stock and fishing mortality within biological safe boundaries. The introduction of a more efficient gear does not threaten the sustainable exploitation as long as fishers comply with the management measures and don’t overshoot their quota. During the transition to pulse trawling the PLH increased their share in the Dutch quota by buying and leasing sole quota from other vessels to compensate for the increased efficiency of the pulse trawl (Turenhout et al. 2016, Hamon et al. 2016).
In the socio-economic domain, the transition to pulse trawling affected the competitive relationship between fishers. Fishers continuously strive to improve their operations in order to increase catches, reduce fishing costs or improve safety and working conditions (Eigaard et al. 2014). Technical innovations, or other causes, may give rise to conflicts between fishers. The differences in economic performance between PT and BT were so big, that once the technique was available for commercial fishing and had demonstrated good results, more beam trawlers wanted to switch. Under pressure to maintain a level playing field within the Dutch fleet, and convinced of the reduction of negative impacts of PT compared to BT based on earlier research findings, the Dutch government sought ways to expand the number of licenses beyond the 5% derogation. This resulted in a large PT fleet in 2014 that changed competitive relationships with other fisheries (Sys et al. 2016; Turenhout et al. 2016; Rijnsdorp et al. 2018b; Vansteenbrugge et al. 2020).
Conflicts between fishers or fisheries are of all times and are an intrinsic component of each fisheries system (de Groot 1984; Spijkers et al. 2019). With the establishment of the EU Common Fisheries Policy in 1982, Member States agreed to share their fishing grounds and agreed on fixed allocation rules of the landings by species and management area. In this management system, fishers are free to compete as long as they don’t exceed their (national) share of the total allowable catch (TAC) and comply with the technical regulations. Despite the relative stability in EU TACs, defined by the fixed allocation rule over the Member States, the autonomous economic and technological development has triggered conflicts between fishers, gear groups and Member States. Conflicts mainly were about economic competition and competition for space, with the PT fleet being able to fish in fishing grounds they could not fish previously (soft grounds). Whilst a local conflict between small scale English fishers and Dutch PT fishers about pulse trawling off the Thames estuary was resolved by an agreement made between the fisheries organisations that the Dutch pulse trawlers would voluntarily stay out of the area, this hardly contributed to conflict resolution; neither did the International Stakeholder Dialogue Meetings set up by the Dutch government.
The way extra licenses were arranged by the Dutch government and the lack of transparency about the pulse settings in the beginning, together with the concerns about the impact of the gear and the experienced competitive disadvantage for many non-pulse fishers undermined trust of stakeholders in the transition. This was the perfect feeding ground for a contra-pulse campaign by Bloom, which turned out to have a lot of effect. The multi-annual research program IAPF and the international stakeholder engagement process set up in response to the stakeholder concerns came too late. Ultimately, and despite published and emerging evidence that the environmental performance and catch efficiency of the PT was superior to the conventional BT, this resulted in a legislative process that resulted in maintaining the ban on electrotrawling (see Sect. "Governance") (Delaney et al. 2023). Nevertheless a small opening is left in the legislation to continue research on electric fishing (Penca 2022).
Given the current scientific evidence presented in this paper, the ban on the use of electricity when catching fish is at odds with the EU objective to improve the ecological and economic sustainability of the fisheries. The replacement of the BT with the PT will contribute to a substantial mitigation of the adverse ecological side effects in the beam trawl fishery for sole, and does not impose any increased risk to the sustainable exploitation of North Sea sole provided that the sole stock is well-managed (ICES 2020b). The pulse trawl technology was developed during a long trajectory where the focus was on a safe and robust gear to catch sole under often harsh commercial conditions. There is still potential to further improve the selectivity of the technology and reduce the remaining side effects. For instance, if the matrix of electrode arrays can be combined with sensors that can detect where a flatfish is buried in the sediment (Nowak and Lankheet 2023), the pulse stimulus could be given to the electrode pair to cramp and catch the detected fish. By adjusting the design of the electrode array so that they only expose the buried flatfish could mitigate pulse-induced injuries if the gear is operated in areas and seasons where these injuries become substantial.
Electrical stimulation has also potential for other fisheries (Soetaert et al. 2015b) as well as other regions where there are rising concerns about trawling impacts on seabed habitats and biota (including those resulting from essential research surveys) and general animal welfare. The application of a low-frequency pulse in the beam trawl fishery for brown shrimps (Crangon crangon) was shown to reduce the often substantial bycatch of fish and other benthic organisms (Polet et al. 2005; Verschueren et al. 2019). Also in the fishery for razor clams (Ensis spp) the use of electricity has been shown an effective alternative for the use of hydraulic dredges that are known to penetrate deep into the sediment and have a large impact on the benthic ecosystem (Murray et al. 2016; Hiddink et al. 2017). Further studies on how fish respond to electrical stimuli may further guide the development of innovative gear in other fisheries.
Given the potential for improving the sustainability of fisheries and the controversial nature of its use, the case study on pulse trawling demonstrates that it is of paramount importance to involve stakeholders very early on in the process to ensure co-development of innovation and a shared vision of the environmental or governance questions that need to be addressed (Delaney et al. 2023). However, it is important to remain aware that shared understanding and co-production of knowledge throughout the process does not automatically guarantee successful science-based introduction of innovations, as the political decision-making process is also influenced by lobby, political interests and (potentially) court cases.
Data availability
Data sharing is not applicable to this article. Primary VMS-data and catch and effort data of the mandatory logbook of the Dutch beam trawl fishery are subject to confidentiality agreements. One should contact Sieto Verver, Head of the Centre for Fisheries Research (sieto.verver@wur.nl) for permission to use these data.
Notes
At the start of the pulse trawling period the pulse settings were slightly different. Pulse amplitude: 60 V; power per meter gear width: 0.7–1 W.m−1; distance between the electrode arrays: 32.5 cm; HFK pulse type: PAC.
References
Anon (2017) Getuigenissen over de terugvallende visvangsten in de zuidelijke Noordzee. LIFSN, 7 September 2017. pp 7
Beare D, Rijnsdorp AD, Blaesberg M, Damm U, Egekvist J, Fock H, Kloppmann M, Röckmann C, Schroeder A, Schulze T, Tulp I, Ulrich C, van Hal R, van Kooten T, Verweij M (2013) Evaluating the effect of fishery closures: lessons learnt from the Plaice Box. J Sea Res 84:49–60
Beaumont WRC (2016) Electricity in fish research and management: theory and practice, 2nd edn. Wiley, Chichester, p 192
Bergman MJN, Meesters EH (2020) First indications for reduced mortality of non-target invertebrate benthic megafauna after pulse beam trawling. ICES J Mar Sci 77:846–857
Bloom (2018) Electric 'pulse' fishing: why it should be banned. http://www.bloomassociation.org/en/wp-content/uploads/2018/01/electric-fishing-advocacy.pdf
Boute PG (2022) Effects of electrical stimulation on marine organisms. PhD thesis, Wageningen University. PhD, Wageningen, Wageningen
Boute PG, Hagmayer A, Smid K, Pieters RP, Lankheet M (2024) Behavioural response thresholds of marine fish species for pulsed electric fields. Front Mar Sci 10:1286149
Boute PG, Rijnsdorp AD, van Leeuwen JL, Versteeg WSM, Pieters RP, Lankheet MJ (2022) Internal injuries in whiting (Merlangius merlangus) caught by tickler-chain and pulse-trawl gears. Fish Res 253:106351
Boute PG, Rijnsdorp AD, van Leeuwen JL, Pieters RPM, Lankheet MJ (2023) Internal injuries in marine fishes caught in beam trawls using electrical versus mechanical stimulations. ICES J Mar Sci 80:1367–1381
Boute PG, Soetaert M, Reid Navarro JA, Lankheet MJ (2021) Effects of electrical pulse stimulation on behaviour and survival of marine benthic invertebrates. Front Mar Sci 7:592650
Bunn N, Fox C, Webb T (2000) A literature review of studies on fish egg mortality: implications for the estimation of spawning stock biomass by the annual egg production method. Science Series Technical Report. CEFAS, Lowestoft. 111, p 37. Science Series Technical Report. CEFAS, Lowestoft., Lowestoft
Collie JS, Hall SJ, Kaiser MJ, Poiner IR (2000) A quantitative analysis of fishing impacts on shelf-sea benthos. J Anim Ecol 69:785–798
De Borger E, Tiano J, Braeckman U, Rijnsdorp AD, Soetaert K (2021) Impact of bottom trawling on sediment biogeochemistry: a modelling approach. Biogeosciences 18:2539–2557
de Groot SJ (1984) The impact of bottom trawling on benthic fauna of the North Sea. Ocean Manage 9:177–190
de Haan D, Burggraaf D (2018) Field strength profile in and above the seabed as reference to pulse trawl fishing on Dover sole (Solea solea). Wageningen Marine Research report C022/18. Wageningen Marine Research report C022/18. Wageningen Marine Research, IJmuiden
de Haan D, Fosseidengen JE, Fjelldal PG, Burggraaf D (2011) The effect of electric pulse stimulation to juvenile cod and cod of commercial landing size. IMARES Report Number C141(11):44
de Haan D, Fosseidengen JE, Fjelldal PG, Burggraaf D, Rijnsdorp AD (2016) Pulse trawl fishing: characteristics of the electrical stimulation and the effect on behaviour and injuries of Atlantic cod (Gadus morhua). ICES J Mar Sci 73:1557–1569
de Haan D, Haenen O, Chen C, Hofman A, van Es Y, Burggraaf D, Blom E (2015) Pulse trawl fishing: the effects on dab (Limanda limanda). IMARES Wageningen UR. Report number C171/14. 43 pp.
de Haan D, van Marlen B, Kristiansen TS, Fosseidengen JE (2008) The effect of pulse stimulation on biota–Research in relation to ICES advice–Progress report on the effects on cod. Imares Report number C098/08
de Haan D, van Marlen B, Velzenboer I, van der Heul J, van der Vis H (2009) The effects of pulse stimulation on biota–Research in relation to ICES advice–Effects on dogfish. IMARES Report C105/09. p 32.
Delaney A, Reid DG, Zimmermann C, Kraan M, Steins NA, Kaiser MJ (2023) Socio-technical approaches are needed for innovation in fisheries. Rev Fish Sci Aquac 31:161–179
Depestele J, Degrendele K, Esmaeili M, Ivanović A, Kröger S, O’Neill FG, Parker R, Polet H, Roche M, Teal LR, Vanelslander B, Rijnsdorp AD (2019) Comparison of mechanical disturbance in soft sediments due to tickler-chain SumWing trawl vs. electro-fitted PulseWing trawl. ICES J Mar Sci 76:312–329
Depestele J, Ivanović A, Degrendele K, Esmaeili M, Polet H, Roche M, Summerbell K, Teal LR, Vanelslander B, O’Neill FG (2016) Measuring and assessing the physical impact of beam trawling. ICES J Mar Sci 73:i15–i26
Desender M, Chiers K, Polet H, Verschueren B, Saunders JH, Ampe B, Mortensen A, Puvanendran V, Decostere A (2016) Short-term effect of pulsed direct current on various species of adult fish and its implication in pulse trawling for brown shrimp in the North Sea. Fish Res 179:90–97
Desender M, Decostere A, Adriaens D, Duchateau L, Mortensen A, Polet H, Puvanendran V, Verschueren B, Chiers K (2017a) Impact of pulsed direct current on Embryos, Larvae, and Young Juveniles of Atlantic Cod and its implications for electrotrawling of brown shrimp. Mar Coastal Fish 9:330–340
Desender M, Dumolein L, Duchateau L, Adriaens D, Delbare D, Polet H, Chiers K, Decostere A (2018) Pulse trawling: the impact of pulsed direct current on early life stages of sole Solea solea. N Am J Fish Manage 38:432–438
Desender M, Kajiura S, Ampe B, Dumolein L, Polet H, Chiers K, Decostere A (2017b) Pulse trawling: evaluating its impact on prey detection by small-spotted catshark (Scyliorhinus canicula). J Exp Mar Biol Ecol 486:336–343
Dijkgraaf S, Kalmijn AJ (1963) Untersuchungen über die Funktion der Lorenzinischen Ampullen an Haifischen. Z Vgl Physiol 47:438–456
EC (1998) Council Regulation (EC) No 850/98 of 30 March 1998 for the conservation of fishery resources through technical measures for the protection of juveniles of marine organisms. J Eur Union, OJ L 125, 27.4.1998, p 36
EC (2004) Communication from the commission to the council and the european parliament “promoting environmentally-friendly fishing methods: the role of technical conservation measures.” Nr Commission Document COM 2004:438
EC (2013) Regulation (EU) No 1380/2013 of the European parliament and of the council of 11 December 2013 on the common fisheries policy, amending council regulations (EC) No 1954/2003 and (EC) No 1224/2009 and repealing Council Regulations (EC) No 2371/2002 and (EC. Official Journal of the European Union, L 354/22.
Eigaard OR, Bastardie F, Breen M, Dinesen GE, Hintzen NT, Laffargue P, Mortensen LO, Nielsen JR, Nilsson HC, O’Neill FG, Polet H, Reid DG, Sala A, Sköld M, Smith C, Sørensen TK, Tully O, Zengin M, Rijnsdorp AD (2016) Estimating seabed pressure from demersal trawls, seines, and dredges based on gear design and dimensions. ICES J Mar Sci 73:i27–i43
Eigaard OR, Marchal P, Gislason H, Rijnsdorp AD (2014) Technological development and fisheries management. Rev Fish Sci Aquac 22:156–174
Emery L (1984) The physiological effects of electrofishing. California-Nevada Wildlife Trans 1984(1984):59–72
Ford J, Muiruri E, Skirrow R, Fox M, Garcia C, Bremner J, Catchpole T (2019) A study to investigate the potential ecological impacts of pulse trawling. Centre for Environment, Fisheries & Aquaculture Science, Lowestoft, England
Gillis DM (2003) Ideal free distributions in fleet dynamics: a behavioral perspective on vessel movement in fisheries analysis. Can J Zool 81:177–187
Gray CA, Kennelly SJ (2018) Bycatches of endangered, threatened and protected species in marine fisheries. Rev Fish Biol Fish 28:521–541
Haasnoot T, Kraan M, Bush SR (2016) Fishing gear transitions: lessons from the Dutch flatfish pulse trawl. ICES J Mar Sci 73:1235–1243
Hamon KG, de Vos B, Poos JJ, Rijnsdorp AD, Verlé K, Kinds A, Polet H (2016) Deliverable 1.3: the economics of technological innovations to mitigate ecosystem effects of fishing: the pulse trawl in the North Sea. Benthic Ecosystem Fisheries Impact Study (BENTHIS): Ijmuiden. 26 pp.
Hatcher A, Frere J, Pascoe S, Robinson K (2002) “Quota-hopping” and the foreign ownership of UK fishing vessels. Mar Policy 26:1–11
Hiddink JG, Jennings S, Sciberras M, Bolam S, McConnaughey RA, Mazor T, Hilborn R, Collie JS, Pitcher R, Parma AM, Suuronen P, Kaiser MJ, Rijnsdorp AD (2019) The sensitivity of benthic macroinvertebrates to bottom trawling impacts using their longevity. J Appl Ecol 56:1075–1084
Hiddink JG, Jennings S, Sciberras M, Szostek CL, Hughes KM, Ellis N, Rijnsdorp AD, McConnaughey RA, Mazor T, Hilborn R, Collie JS, Pitcher CR, Amoroso RO, Parma AM, Suuronen P, Kaiser MJ (2017) Global analysis of depletion and recovery of seabed biota after bottom trawling disturbance. Proc Natl Acad Sci 114:8301–8306
Hintzen NT, Aarts G, Poos JJ, Van der Reijden KJ, Rijnsdorp AD (2021) Quantifying habitat preference of bottom trawling gear. ICES J Mar Sci 78:172–184
Horwood J (1993) The bristol channel sole (Solea solea (L))—A Fisheries case-study. Adv Mar Biol Book 29:215–367
ICES (2006) Answer to special request on pulse trawl electric fishing gear. International Council for the Exploration of the Sea, Copenhagen
ICES (2018) Report of the working group on electric trawling (WGELECTRA). 17–19 April 2018. IJmuiden, The Netherlands. International for the Exploration of the Sea, Copenhagen
ICES (2020a) ICES Working Group on Electrical Trawling (WGELECTRA). ICES Scientific Reports. 2:37. p. 108 https://doi.org/10.17895/ices.pub.6006.
ICES (2020b) Request of the Netherlands on the ecosystem and environmental impacts of pulse trawling for the sole (Solea solea) fishery in the North Sea. In Report of the ICES Advisory Committee, 2020. ICES Advice 2020, sr.2020.03. https://doi.org/10.17895/ices.advice.6020.
ICES (2023) Working group on the assessment of demersal stocks in the North Sea and Skagerrak (WGNSSK). ICES Scientific Reports. 5:39. p. 1256. https://doi.org/10.17895/ices.pub.22643143.
Kelleher K (2005) Discards in the world’s marine fisheries: an update, vol 470. Food and Agriculture Organisation, Rome
Kraan M, Schadeberg A (2018) International Stakeholder Dialogue on Pulse Fisheries. Report of the third dialogue meeting, Amsterdam, June 19, 2018. Wageningen, Wageningen Marine Research (University & Research centre), Wageningen Marine Research report C111/18.
Kraan M, Trapman BK, Rasenberg MMM (2015) Perceptions of European stakeholders of pulse fishing. IMARES Report number C098/15. 44 pp.
Le Manach F, Bisiaux L, Villasante S, Nouvian C (2019) Public subsidies have supported the development of electric trawling in Europe. Mar Policy 104:225–231
Leggett WC, Deblois E (1994) Recruitment in marine fishes—Is it regulated by starvation and predation in the egg and larval stages. Neth J Sea Res 32:119–134
Linnane A, Ball B, Munday B, van Marlen B, Bergman M, Fonteyne R (2000) A review of potential techniques to reduce the environmental impact of demersal trawls. Irish Fish Investig 7:7–39
Lowerre-Barbieri S, DeCelles G, Pepin P, Catalán IA, Muhling B, Erisman B, Cadrin SX, Alós J, Ospina-Alvarez A, Stachura MM, Tringali MD, Burnsed SW, Paris CB (2017) Reproductive resilience: a paradigm shift in understanding spawner-recruit systems in exploited marine fish. Fish Fish 18:285–312
McConnaughey RA, Hiddink JG, Jennings S, Pitcher CR, Kaiser MJ, Suuronen P, Sciberras M, Rijnsdorp AD, Collie JS, Mazor T, Amoroso RO, Parma AM, Hilborn R (2020) Choosing best practices for managing impacts of trawl fishing on seabed habitats and biota. Fish Fish 21:319–337
Morin M (2000) The fisheries resources in the European Union.: the distribution of TACs: principle of relative stability and quota-hopping. Mar Policy 24:265–273
Murray F, Copland P, Boulcott P, Robertson M, Bailey N (2016) Impacts of electrofishing for razor clams (Ensis spp.) on benthic fauna. Fish Res 174:40–46
Nowak L, Lankheet M (2023) Bioinspired flatfish detection using electrical impedance measurements. Bioinspir Biomim 18:064002
Oberle FKJ, Storlazzi CD, Hanebuth TJJ (2016) What a drag: quantifying the global impact of chronic bottom trawling on continental shelf sediment. J Mar Syst 159:109–119
Parker RWR, Tyedmers PH (2015) Fuel consumption of global fishing fleets: current understanding and knowledge gaps. Fish Fish 16:684–696
Paschen M, Richter U, Kopnick W (2000) Trawl penetration in the seabed (TRAPESE). Final Report EC-Study Contract No 96–006 University of Rostock, Rostock, Germany:150
Penca J (2022) Science, precaution and innovation for sustainable fisheries: the judgement by the Court of Justice of the EU regarding the electric pulse fishing ban. Mar Policy 135:104864
Penfield W, Jasper H (1954) Epilepsy and the functional anatomy of the human brain. Little Brown & Co, Boston
Pitcher CR, Ellis N, Jennings S, Hiddink JG, Mazor T, Kaiser MJ, Kangas MI, McConnaughey RA, Parma AM, Rijnsdorp AD, Suuronen P, Collie JS, Amoroso R, Hughes KM, Hilborn R (2017) Estimating the sustainability of towed fishing-gear impacts on seabed habitats: a simple quantitative risk assessment method applicable to data-limited fisheries. Methods Ecol Evol 8:472–480
Polet H, Delanghe F, Verschoore R (2005) On electrical fishing for brown shrimp (Crangon crangon)—II. Sea Trials Fish Res 72:13–27
Poos JJ, Hintzen NT, van Rijssel J, Rijnsdorp AD (2020) Efficiency changes in bottom trawling for flatfish species as a result of the replacement of mechanical stimulation by electric stimulation. ICES J Mar Sci 77:2635–2645
Pusceddu A, Fiordelmondo C, Polymenakou P, Polychronaki T, Tselepides A, Danovaro R (2005) Effects of bottom trawling on the quantity and biochemical composition of organic matter in coastal marine sediments (Thermaikos Gulf, northwestern Aegean Sea). Cont Shelf Res 25:2491–2505
Rijnsdorp AD, Aarts G, Hintzen NT, van Rijssel JC, Winter AM, Poos JJ (2022) Fishing tactics and the effect of resource depletion and interference during the exploitation of local patches of flatfish. ICES J Mar Sci 79:2093–2106
Rijnsdorp AD, Batsleer J, Molenaar P (2021a) The effect of electrical stimulation on the footrope and cod-end selection of a flatfish bottom trawl. Fish Res 243:106104
Rijnsdorp AD, Bolam SG, Garcia C, Hiddink JG, Hintzen N, van Denderen PD, van Kooten T (2018a) Estimating the sensitivity of seafloor habitats to disturbance by bottom trawl fisheries based on the longevity of benthic fauna. Ecol Appl 28:1302–1312
Rijnsdorp AD, Boute P, Tiano J, Lankheet M, Soetaert K, Beier U, de Borger E, Hintzen N.T., Molenaar P, Polet H, Poos JJ, Schram E, Soetaert M, Van Overzee H, van de Wolfshaar K, Van Kooten T (2020a) The implications of a transition from tickler chain beam trawl to electric pulse trawl on the sustainability and ecosystem effects of the fishery for North Sea sole: an impact assessment. Wageningen University & Research Report C037/20. Wageningen University & Research, IJmuiden, The Netherlands
Rijnsdorp AD, Depestele J, Eigaard OR, Hintzen NT, Ivanovic A, Molenaar P, O’Neill FG, Polet H, Poos JJ, van Kooten T (2020b) Mitigating seafloor disturbance of bottom trawl fisheries for North Sea sole Solea solea by replacing mechanical with electrical stimulation. PLoS ONE 8(4):e61357
Rijnsdorp AD, Depestele J, Molenaar P, Eigaard OR, Ivanoviċ A, O’Neill FG (2021b) Sediment mobilisation by bottom trawls: a model approach applied to the Dutch North Sea beam trawl fishery. ICES J Mar Sci 78:1574–1586
Rijnsdorp AD, Hiddink JG, van Denderen PD, Hintzen NT, Eigaard OR, Valanko S, Bastardie F, Bolam S, Boulcot P, Egekvist J, Garcia C, van Hoey G, Jonsson P, Laffargue P, Nielsen JR, Piet GJ, Sköld M, van Kooten T (2020c) Different bottom trawl fisheries have a differential impact on the status of the North Sea seafloor habitats. ICES J Mar Sci 77:1772–1786
Rijnsdorp AD, van Rijssel J, Hintzen NT (2018b) Declining catch rates of small scale fishers in the southern North Sea in relation to the pulse transition in the beam trawl fleet. Wageningen Marine Research Report C051/18.
Rytwinski T, Taylor JJ, Donaldson LA, Britton JR, Browne DR, Gresswell RE, Lintermans M, Prior KA, Pellatt MG, Vis C (2019) The effectiveness of non-native fish removal techniques in freshwater ecosystems: a systematic review. Environ Rev 27:71–94
Sala A, Damalas D, Labanchi L, Martinsohn J, Moro F, Sabatella R, Notti E (2022) Energy audit and carbon footprint in trawl fisheries. Scientific Data 9:428
Schram E, Molenaar P (2018) Discards survival probabilities of flatfish and rays in North Sea pulse-trawl fisheries. Wageningen University & Research Report C037/18
Schram E, Molenaar P, de Koning S, Rijnsdorp AD (2022a) A transdisciplinary approach towards studying direct mortality among demersal fish and benthic invertebrates in the wake of pulse trawling. Front Mar Sci 9:907192
Schram E, Molenaar P, Goedhart PW, Poos JJ (2023) Effects of environmental conditions and catch processing on survival probability of plaice discards in the North Sea pulse trawl fishery. PLoS ONE 18:e0287020
Schram E, Molenaar P, Kleppe R, Rijnsdorp A (2020) Condition and survival of discards in tickler chain beam trawl fisheries. Wageningen Marine Research report C034/20.
Schram E, Molenaar P, Soetaert M, Burggraaf D, Boute PG, Lankheet MJ, Rijnsdorp AD (2022b) Effect of electrical stimulation used in the pulse trawl fishery for common sole on internal injuries in sandeels. ICES J Mar Sci 79:1561–1568
Schram E, van de Pol L, Molenaar P, van Mens A, Bleeker K, Gazi KM, Batsleer J (2023b) Survival probabilities of thornback and spotted rays discarded by beam trawl and flyshoot fisheries. Wageningen Marine Research Report C018/23.
Schram E, van de Pol L, van Mens A, van Dalen P, Suykerbuyk W, Cornelisse S, Mattens A, Molenaar P (2023c) Survival probabilities of plaice, sole and turbot discarded by beam trawl and flyshoot fisheries. Wageningen Marine Research Report C059/23.
Sciberras M, Hiddink JG, Jennings S, Szostek CL, Hughes KM, Kneafsey B, Clarke L, Ellis N, Rijnsdorp AD, McConnaughey RA, Hilborn R, Collie JS, Pitcher CR, Amoroso RO, Parma AM, Suuronen P, Kaiser MJ (2018) Response of benthic fauna to experimental bottom fishing: a global meta-analysis. Fish Fish 19:698–715
Smaal AC, Brummelhuis E (2005) Explorative studies of the impact of an electric fishing field on macrobenthos. RIVO C089(05):15p
Snyder DE (2003a) Electrofishing and its harmful effects on fish. Book Information and Technology Report USGS/BRD/ITR–2003–0002, U.S. Geological Survey Biological Resources Division. U.S. Government Printing Office, Denver, CO
Snyder DE (2003) Invited overview: conclusions from a review of electrofishing and its harmful effects on fish. Rev Fish Biol Fish 13:445–453
Soetaert K, Herman PM, Middelburg JJ (1996) A model of early diagenetic processes from the shelf to abyssal depths. Geochim Cosmochim Acta 60:1019–1040
Soetaert M (2015) Electrofishing: exploring the safety range of electric pulses for marine species and its potential for further innovation. PhD, Gent.
Soetaert M, Boute PG, Beaumont WRC (2019) Guidelines for defining the use of electricity in marine electrotrawling. ICES J Mar Sci 76:1994–2007
Soetaert M, Chiers K, Duchateau L, Polet H, Verschueren B, Decostere A (2015a) Determining the safety range of electrical pulses for two benthic invertebrates: brown shrimp (Crangon crangon L.) and ragworm (Alitta virens S.). ICES J Mar Sci 72:973–980
Soetaert M, de Haan D, Verschueren B, Decostere A, Puvanendran V, Saunders J, Polet H, Chiers K (2016a) Atlantic Cod show a highly variable sensitivity to electric-induced spinal injuries. Mar Coast Fish 8:412–424
Soetaert M, Decostere A, Polet H, Verschueren B, Chiers K (2015b) Electrotrawling: a promising alternative fishing technique warranting further exploration. Fish Fish 16:104–124
Soetaert M, Decostere A, Verschueren B, Saunders J, Van Caelenberge A, Puvanendran V, Mortensen A, Duchateau L, Polet H, Chiers K (2016b) Side-effects of electrotrawling: exploring the safe operating space for Dover sole (Solea solea L.) and Atlantic cod (Gadus morhua L.). Fish Res 177:95–103
Soetaert M, Verschueren B, Chiers K, Duchateau L, Polet H, Decostere A (2016c) Laboratory study of the impact of repetitive electrical and mechanical stimulation on brown shrimp Crangon crangon. Mar Coast Fish 8:404–411
Soetaert M, Verschueren B, Decostere A, Saunders J, Polet H, Chiers K (2018) No injuries in European Sea Bass tetanized by pulse stimulation used in electrotrawling. N Am J Fish Manage 38:247–252
Spijkers J, Singh G, Blasiak R, Morrison TH, Le Billon P, Österblom H (2019) Global patterns of fisheries conflict: forty years of data. Global Environ Change 57:101921
STECF (2006) 23rd report of the scientific, technical and economic committee for fisheries, Barza d’Ispra, 6–10 November 2006. Commission of the European Communities, Brussels
Steins N (2018) Bijeenkomst passieve visserij rond vragen over pulsvisserij. Notulen bijeenkomst 16 maart 2018. Zeehaven IJmuiden. Wageningen University & Research. 1808391.NSt.mb. p 8.
Steins NA, Smith S, Strietman WJ, Trapman B, Kraan M (2017) International Stakeholder Dialogue on Pulse Fisheries. Report of the second dialogue meeting, Amsterdam 20 January 2017. Wageningen Marine Research (Wageningen, University & Research centre), Wageningen Marine Research report C016/17, 145 pp.
Sternin VG, Nikonorov IV, Bumeister YK (1976) Electrical fishing. Theory and practice. Keter Publishing House Jerusalem Ltd., Jerusalem
Stewart PAM (1975) Catch selectivity by electrical fishing systems. ICES J Mar Sci 36:106–109
Stewart PAM (1977) A study of the response of flatfish (Pleuronectidae) to electrical stimulation. ICES J Mar Sci 37:123–129
Sys K, Poos JJ, Van Meensel J, Polet H, Buysse J (2016) Competitive interactions between two fishing fleets in the North Sea. ICES J Mar Sci 73:1485–1493
Tiano J, Depestele J, Van Hoey G, Fernandes J, van Rijswijk P, Soetaert K (2022) Trawling effects on biogeochemical processes are mediated by fauna in high energy biogenic reef–inhabited coastal sediments. Biogeosci Discuss 2022:1–36
Tiano JC, De Borger E, O’Flynn S, Cheng CH, van Oevelen D, Soetaert K (2021) Physical and electrical disturbance experiments uncover potential bottom fishing impacts on benthic ecosystem functioning. J Exp Mar Biol Ecol 545:151628
Tiano JC, van der Reijden KJ, O’Flynn S, Beauchard O, van der Ree S, van der Wees J, Ysebaert T, Soetaert K (2020) Experimental bottom trawling finds resilience in large-bodied infauna but vulnerability for epifauna and juveniles in the Frisian Front. Mar Environ Res 159:104964
Tiano JC, Witbaard R, Bergman MJN, van Rijswijk P, Tramper A, van Oevelen D, Soetaert K (2019) Acute impacts of bottom trawl gears on benthic metabolism and nutrient cycling. ICES J Mar Sci 76:1917–1930
Turenhout MNJ, Zaalmink BW, Strietman WJ, Hamon KG (2016) Pulse fisheries in the Netherlands; Economic and spatial impact study. Wageningen Economic Research, Report 2016–104. Wageningen
Tyedmers PH, Watson R, Pauly D (2005) Fueling global fishing fleets. AMBIO: J Human Environ 34:635–638
Uhlman SS, Ulrich C, Kennelly SJ (eds) (2019) The European landing obligation reducing discards in complex, multi-species and multi-jurisdictional fisheries. Springer, Cham
Uhlmann SS, Ampe B, Van Bogaert N, Vanden Berghe C, Vanelslander B (2021) Flatfish survivors have tales to tell: cold seawater and reduced deployment duration contribute to the survival of European plaice (Pleuronectes platessa) discarded by Belgian beam trawlers. Fish Res 240:105966
Uhlmann SS, Theunynck R, Ampe B, Desender M, Soetaert M, Depestele J (2016) Injury, reflex impairment, and survival of beam-trawled flatfish. ICES J Mar Sci 73:1244–1254
van Balsfoort G, IJlstra T, Steins N, Vroegop F (2006) Vissen met tegenwind. Advies Task Force Duurzame Noordzeevisserij. Productschap Vis, Rijswijk
van Beek FA, van Leeuwen PI, Rijnsdorp AD (1990) On the survival of plaice and sole discards in the Otter-Trawl and Beam-trawl fisheries in the North-Sea. Neth J Sea Res 26:151–160
van de Velde S, Van Lancker V, Hidalgo-Martinez S, Berelson WM, Meysman FJ (2018) Anthropogenic disturbance keeps the coastal seafloor biogeochemistry in a transient state. Sci Rep 8:1–10
van de Wolfshaar KE, van Denderen PD, Schellekens T, van Kooten T (2020) Food web feedbacks drive the response of benthic macrofauna to bottom trawling. Fish Fish 21:962–972
van de Wolfshaar KE, van Kooten T, Rijnsdorp AD (2020b) Lethal and non-lethal effects of trawling on the benthic invertebrate food web. WMR report C011/20. https://doi.org/10.18174/514206.
van der Reijden KJ, Koop L, O’Flynn S, Garcia S, Bos O, van Sluis C, Maaholm DJ, Herman PMJ, Simons DG, Olff H, Ysebaert T, Snellen M, Govers LL, Rijnsdorp AD, Aguilar R (2019) Discovery of Sabellaria spinulosa reefs in an intensively fished area of the Dutch Continental Shelf, North Sea. J Sea Res 144:85–94
van der Reijden KJ, Molenaar P, Chen C, Uhlmann SS, Goudswaard PC, van Marlen B (2017) Survival of undersized plaice (Pleuronectes platessa), sole (Solea solea), and dab (Limanda limanda) in North Sea pulse-trawl fisheries. ICES J Mar Sci 74:1672–1680
van Dijk TAGP, van Dalfsen JA, Van Lancker V, van Overmeeren RA, van Heteren S, Doornenbal PJ, Harris PT, Baker EK (2012) 13 - benthic habitat variations over Tidal Ridges, North Sea, the Netherlands. In: Harris PT, Baker EK (eds) Seafloor Geomorphology as Benthic Habitat. Elsevier, London
van Hoof L, Steins NA, Smith S, Kraan M (2020) Change as a permanent condition: a history of transition processes in Dutch North Sea fisheries. Mar Policy 122:104245
Van Marlen B, Bergman M, Groenewold S, Fonds M (2001) Research on diminishing impact in demersal trawling–The experiments in The Netherlands. ICES CM2001/R:09:54
van Marlen B, de Haan D (1988) Elektrische stimulering van platvis (verleden, heden en toekomst). Rijks Instituut voor Visserij Onderzoek, TO 88–06. p. 98
van Marlen B, de Haan D, van Gool A, Burggraaf D (2009) The effect of pulse stimulation on marine biota—Research in relation to ICES Advice—Progress report on the effects on benthic invertebrates. IMARES Report C103/09. 53 pp.
van Marlen B, Wiegerinck JAM, van Os-Koomen E, van Barneveld E (2014) Catch comparison of flatfish pulse trawls and a tickler chain beam trawl. Fish Res 151:57–69
Van Overzee HMJ, Rijnsdorp AD, Poos JJ (2023) Changes in catch efficiency and selectivity in the beam trawl fishery for sole when mechanical stimulation is replaced by electrical stimulation. Fish Res 260(106603):1–10
Vansteenbrugge L, Sys K, Nimmegeers S, Vandecasteele L, Vanelslander B, Vandemaele S, Vanderperren E, Polet H, Torreele E (2020) Pulsvisserij Vlaamse kust – Deel 1. Instituut voor Landbouw- Visserij- en Voedingsonderzoek (ILVO), Eenheid Dier – Visserij en Aquatische productie
Veldhuizen LJL, Berentsen PBM, de Boer IJM, van de Vis JW, Bokkers EAM (2018) Fish welfare in capture fisheries: a review of injuries and mortality. Fish Res 204:41–48
Verschueren B, Lenoir H, Soetaert M, Polet H (2019) Revealing the by-catch reducing potential of pulse trawls in the brown shrimp (Crangon crangon) fishery. Fish Res 211:191–203
Yu C, Chen Z, Chen L, He P (2007) The rise and fall of electrical beam trawling for shrimp in the East China Sea: technology, fishery, and conservation implications. ICES J Mar Sci 64:1592–1597
Zaalmink W, Hoekstra G, Mol A, Strietman WJ (2018) Sociaal-economische gevolgen van een totaalverbod op pulsvisserij voor de Nederlandse visserijsector. Wageningen, Wageningen Economic Research, Nota 2018–044. p. 36
Acknowledgements
We are grateful to Dr Christopher Zimmerman for correcting Figure 1 and to Dr Lennert van de Pol for updating the VMS and catch and effort data sets of the Dutch beam trawl fleet. We thank the fishers who have been involved in collecting data for the studies reviewed in this paper.
Funding
The multi-annual Impact Assessment Pulse Fishery (IAPF) study was funded by the European Maritime and Fisheries Fund of the European Union (contract number 1300021172). Research into social and governance aspects of pulse trawling was funded through the Policy Support Research Theme Nature Inclusive Fisheries of the Dutch Ministry for Agriculture, Nature and Food Quality (Project Nr. BO-43–023.02–004).
Author information
Authors and Affiliations
Contributions
This study was conducted as part of the IAPF research project. The research questions and research design was developed by ADR, HP, ML and KS. PB, JT, DdH, ES, MS and ADR contributed to the review of the ecological literature. NS and MK reviewed the governance literature. The article was written by ADR with contributions of PB, JT, ML, ES, NS, MK. All authors evaluated the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rijnsdorp, A.D., Boute, P.G., Tiano, J.C. et al. Electrotrawling can improve the sustainability of the bottom trawl fishery for sole: a review of the evidence. Rev Fish Biol Fisheries (2024). https://doi.org/10.1007/s11160-024-09867-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11160-024-09867-x