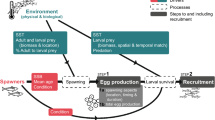
Abstract
Understanding the drivers behind fluctuations in fish populations remains a key objective in fishery science. Our predictive capacity to explain these fluctuations is still relatively low, due to the amalgam of interacting bottom-up and top-down factors, which vary across time and space among and within populations. Gaining a mechanistic understanding of these recruitment drivers requires a holistic approach, combining field, experimental and modelling efforts. Here, we use the Western Baltic Spring-Spawning (WBSS) herring (Clupea harengus) to exemplify the power of this holistic approach and the high complexity of the recruitment drivers (and their interactions). Since the early 2000s, low recruitment levels have promoted intense research on this stock. Our literature synthesis suggests that the major drivers are habitat compression of the spawning beds (due to eutrophication and coastal modification mainly) and warming, which indirectly leads to changes in spawning phenology, prey abundance and predation pressure. Other factors include increased intensity of extreme climate events and new predators in the system. Four main knowledge gaps were identified related to life-cycle migration and habitat use, population structure and demographics, life-stage specific impact of multi-stressors, and predator–prey interactions. Specific research topics within these areas are proposed, as well as the priority to support a sustainable management of the stock. Given that the Baltic Sea is severely impacted by warming, eutrophication and altered precipitation, WBSS herring could be a harbinger of potential effects of changing environmental drivers to the recruitment of small pelagic fishes in other coastal areas in the world.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the drivers causing population fluctuations remains a central challenge in fishery science and in ecology in general. For more than a century, fishery scientists have acknowledged that recruitment (the number of young fish entering a stock), can vary up to an order of magnitude from year to year (Hjort 1914). Such variability has important ecological consequences as well as repercussions for fisheries management and a steady supply of wild-caught fish worldwide (Shelton and Mangel 2011). Unfortunately, the capacity to explain past or predict future recruitment is still limited due, in part, to the amalgam of interacting factors and processes impacting year-class success. These factors include parental stock size and structure, condition and number of spawners, and spatio-temporal changes in the environment and prey and predator fields at multiple life stages. The direction and magnitude of these factors is context-dependent, varying across time and space among and within populations (Zimmermann et al. 2019). Moving beyond identification of the drivers through statistical methods would require gaining mechanistic understanding on how they alter fish vital traits (e.g., growth, survival, reproduction, migration behavior). This cause-and-effect knowledge could foster the development of more robust model predictions, improving both short-term tactical fishery advice via integration into stock assessment (Kuparinen et al. 2014; Skern‐Mauritzen et al. 2016), and strategic advice by understanding for instance the medium- to long-term response of fish populations to changing climate conditions (Koenigstein et al. 2016, 2018).
Traditionally, recruitment studies have focused on the effect of bottom-up factors on pre-recruit life stages, that is the impact of abiotic factors placing limits on primary production that propagate to higher trophic levels leading to resource limitation. Prey availability and hydrography (i.e., water currents) were first highlighted as the major factors contributing to larval survival (Hjort 1914; Cushing 1975; Iles and Sinclair 1982). Also, the potential role of maternal effects (i.e., body fat) on offspring fitness was introduced by these early studies (Hjort 1914), a mechanism that we now understand better (Hixon et al. 2014). More recent studies pinpointed the importance of temperature and other long-term climatic changes to year class strength and species shifts (e.g., Takasuka et al. 2007; Cardinale et al. 2009; Alheit et al. 2012). On the other hand, top-down factors were only seldom investigated, although predation has been recognized as (arguably) the major recruitment driver (Bailey and Houde 1989; Houde 2008). Recent research on the topic has focused on understanding the impact of fish and jellyfish predation on early life stages of fish (Pereira et al. 2014; Skaret et al. 2015; Tilves et al. 2018), but there is still relatively little knowledge on predation mortality, partly because it is an aspect difficult to assess (Paradis et al. 1996). Most studies suggest that fast-growing individuals have an increased chance of survival, because they spend less time in early stages particularly vulnerable to predators (Takasuka et al. 2007; but see Robert et al. 2010). Testing these hypotheses empirically in the laboratory or in the field can be challenging due to logistic and practical constraints (Houde 2008). Besides predation, fishing can have a direct effect by decreasing the number of spawners as well as an indirect effect by changing the stock demographics (Hixon et al. 2014). To complicate the picture even more, the strength of the different recruitment drivers may shift from year to year or under long-term ecosystem changes (Möllmann et al. 2008). Therefore, a holistic approach that considers the dynamic interactions of both bottom-up and top-down factors impacting the spawners and early life stages (and their complex, intertwined interactions) is needed to explore changes in recruitment mechanisms (Voss et al. 2012; Hare 2014; Brosset et al. 2020).
Small pelagic fishes are boom-and-bust species that play important roles in a wide range of marine ecosystems (Checkley et al. 2009; Peck et al. 2021). They have an important ecological role as they are in an intermediate trophic position, linking plankton to higher trophic levels and apex predators (as marine mammals and seabirds). Also, they have been the target of fishers for millennia and now constitute some of the biggest fisheries worldwide, with annual landings > 15 million tons (FAO 2018). Stocks of small pelagic fishes experience natural, large climate-driven fluctuations in biomass, which can be aggravated by fishing, leading to stock collapse. Classical examples of such collapses are Pacific sardine (Sardinops sagax) in the California Current in the mid-1940s and Atlantic herring (Clupea harengus) in the North Sea in the early 1970s (Peck et al. 2013, 2021). The intermediate trophic role and the rapid population responses to environmental changes make small pelagic fishes a particularly suitable case for including ecosystem considerations into their management plans (Siple et al. 2021). While such considerations have been incorporated for certain stocks (e.g. temperature in Pacific sardine (Zwolinski et al. 2011)), they were discarded after some years in other stocks due to the non-stationary nature of the relationship (e.g. temperature and zooplankton abundance in Gulf of Riga herring (Arula et al. 2016; ICES 2021a)). These examples highlight the complexity of ecosystem interactions and the challenge of sustainable fisheries management under changing ocean conditions and growing anthropogenic pressures. Thus, there is an urgent need to develop methods to detect changes in productivity and distribution and their underlying mechanisms as a first step to support a more holistic, ecosystem-based fisheries management (Karp et al. 2019; Brosset et al. 2020).
Atlantic herring is one of the best-studied species worldwide (Geffen 2009; Peck et al. 2021). Its commercial importance motivated the research on recruitment mechanisms, leading to seminal recruitment hypotheses, such as the critical period (Hjort 1914) and member-vagrant (Iles and Sinclair 1982), as well as the establishment of the International Council for the Exploration of the Sea (ICES) at the beginning of the twentieth century (Sinclair 2009). Current knowledge supports that herring recruitment is set within the first month(s) of life in North East Atlantic populations, such as those in the Norwegian, North and Baltic Seas (Sætre 2002; Nash and Dickey-Collas 2005; Oeberst et al. 2009). Bottom-up factors, such as changes in temperature and shifts in the abundance and type of prey, have been proposed as potential drivers of recruitment variability in these populations (Cardinale et al. 2009). Other studies have suggested that cannibalism and predation from other forage fishes (e.g., Atlantic mackerel, Scomber scombrus) can have a large impact on larval survival (Allan et al. 2021), but the ultimate consequences for recruitment and the relative contribution of each process is still largely unknown (Corten 2013; Skaret et al. 2015; Garcia et al. 2020). In general, it is now clear that the set of recruitment drivers is highly context-dependent, varying in time and space within and among populations (e.g., Zimmermann et al. 2019).
The present study synthesizes the current knowledge on recruitment drivers in the well-studied Western Baltic Spring–Spawning (WBSS) herring stock, one of the three major herring stocks in the Baltic Sea (Fig. 1). This stock constitutes an excellent case study for several reasons. First, recruitment levels dramatically decreased since the mid-2000s and have remained low since then (Fig. 2) (ICES 2021b). Spawning stock biomass (SSB) has been low since 2007 (Fig. 2), leading ICES to advise closing the fishery since 2019 (ICES 2021b). The condition of this stock has, thus, alarmed fisheries managers and scientists, which has led to more than 40 peer-reviewed papers published since 2011 addressing different aspects of the recruitment dynamics of this stock using a combination of laboratory and field experiments, field monitoring, and modelling approaches. Second, the WBSS herring reproductive strategy (i.e., benthic eggs coupled with restricted larval dispersal) makes this stock especially vulnerable to changing environmental conditions, but at the same time they are an ideal case study to investigate the impact of these changes. Third, the Baltic Sea is characterized by a low species diversity (Möllmann et al. 2008) which, in principle, simplifies the study of trophic interactions impacting recruitment; e.g. the number of predators in the Baltic Sea is limited compared to nearby areas such as the North Sea. Finally, the Baltic Sea is a semi-enclosed brackish sea more severely impacted by climate-driven changes including warming, acidification and altered precipitation patterns compared to many other coastal areas supporting herring stocks (Reusch et al. 2018). Thus, the recent patterns observed in the WBSS herring could be a harbinger of potential effects of changing environmental drivers to the recruitment of small pelagics in other coastal areas in the North East Atlantic. Previous efforts to summarize the literature for this stock were done almost a decade ago (Von Dorrien et al. 2013). Given the extensive knowledge gained in the most recent decade, we provide an up-to-date synthesis of the available literature on recruitment drivers for WBSS herring. Our main goals were to identify knowledge gaps and propose future research avenues to advance our understanding of recruitment drivers in this stock and support management and conservation strategies. We believe this case study exemplifies the power of using a holistic approach to understand the complex array of bottom-up and top-down factors influencing life cycle closure in a marine fish.
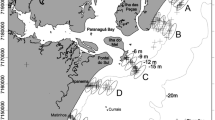
Temporal and spatial characteristics of the life cycle of Western Baltic Spring-Spawning (WBSS) herring. A Map of the Western Baltic showing the spawning (light and dark orange for assumed and confirmed spawning areas, respectively), feeding (yellow) and overwintering grounds (blue) of WBSS herring. Sampling sites in nursery areas are shown for the Rügen Herring Larval Survey in Greifswald Bay (GB) and the GEOMAR-Kiel larval surveys in the Kiel Canal (KC), and Kiel Fjord (KF). The area surveyed during the German Autumn Acoustic Survey (GERAS) is shown in light red. Assumed spawning areas are from Clausen et al. 2015 and confirmed spawning areas from published literature (see “Life cycle and recruitment processes” section). Note, feeding grounds are displayed in yellow for both juveniles (western Baltic) and > 2 year old herring (Skagerrak, Kattegat and North Sea) and these are surveyed in June-July within the HERAS program. B The WBSS herring year cycle, showing the spawning, feeding and overwintering times, as well as the developmental stages during the first year of life in the inner circle and when these fish are sampled in the field (in light red)
Status of Western Baltic Spring–Spawning herring stock: A Times series of the International Council of the Exploration of the Sea (ICES) recruitment index (mean ± 95% CI) and N20 larval index (0 winter-rings) in Greifswald Bay, B spawning stock biomass (SSB) from ICES database including main biomass reference points (including limit—Blim-, precautionary—Bpa- or target reference points—MSY Btrigger-, referring to the Maximum Sustainable Yield; for further details see "Processes influencing spawning biomass and spawning time" section and www.ices.dk), C relationship between N20 larval index (age 0) and juvenile abundance (age 1) from the German Autumn Acoustic Survey (GERAS) with most recent years highlighted in red (for further details on these metrics see "Life cycle and recruitment processes" section), D fishery catches from ICES database
Life cycle and recruitment processes
WBSS herring are migratory and alternate seasonally between their spawning, feeding and overwintering grounds around the Western Baltic, and the Skagerrak-Kattegat (Bekkevold et al. 2015) (Fig. 1). They spawn during spring (peak spawning in March–May) in coastal areas (Fig. 1). In contrast to most other small pelagic fish species that spawn free-floating eggs, herring attach their eggs to demersal substrates such as macrophytes (Balon 1975; Polte and Asmus 2006) or hard substratum, such as pebble or rocks and gravel areas (Neb 1952). WBSS herring spawning and nursery grounds comprise estuaries (such as the Schwentine River or the Warnow River), fjords (such as Flensburg or Kiel Fjord), artificial waterways (such as the Kiel Canal), or shallow bays (such as Greifswald Bay) (Weber, 1971; Klinkhardt, 1986). Despite this diversity, most spawning sites are characterized by shallow waters with low salinity (< 12), and the presence of submerged vegetation. These shallow waters have high primary productivity and warm quickly in spring, promoting the development of large quantities of mesozooplankton, especially calanoid copepods such as Eurytemora affinis and Acartia tonsa, which are important prey for herring larvae (Paulsen et al. 2016).
The two most-studied spawning grounds and nursery areas for WBSS herring are Greifswald Bay and Kiel Canal. Greifswald Bay is traditionally considered the major spawning site for WBSS herring, although other areas can also be important in some years (Moll, 2018; Moll et al. 2019). This Bay is very shallow (mean depth of 5.8 m), has a salinity ranging between 6 and 8, and weak tidal currents due to negligible lunar tides (< 10 cm). Larvae stay within the Bay from hatch until they metamorphose into juveniles, showing stage-specific and multidirectional habitat selection between upper littoral and pelagic habitats (Polte et al. 2017). Therefore, Greifswald Bay can be considered as a “natural mesocosm”. The Kiel Canal, on the other hand, is an artificial waterway with an average width of 100 m, a mean depth of 11 m and a salinity ranging from 5 to 12. Within one year after its opening (1895), large herring schools and young herring larvae were captured in the Canal (Hinkelmann, 1897). The water column in the Kiel Canal is constantly mixed due to intense ship traffic (https://www.kiel-canal.de/) and is a suitable habitat to examine prey effects on larval fish, due to its very confined space and weak currents (Paulsen et al. 2014a,b). Eurytemora affinis is the dominant calanoid copepod species in the Kiel Canal and in the Greifswald Bay, but their abundance varies through the season alternating with those of Acartia tonsa (Diekmann et al. 2012; Paulsen et al. 2016).
Herring juveniles leave the nursery areas in late spring/early summer and seem to remain in the Western Baltic Sea area the first two years (Fig. 1) (Nielsen et al. 2001). Once they are adults (age group 3 +), they use the Skagerrak summer feeding grounds after spawning, where they mix with adjacent herring populations from the North Sea and Eastern Baltic Sea (Fig. 1) (Nielsen et al. 2001; Clausen et al. 2015; Berg et al. 2017). Estimates for biomass, abundance, length structure and maturity proportion of WBSS adults in the summer feeding grounds are obtained within the ICES-coordinated Herring Acoustic Survey in the Skagerrak and Kattegat (HERAS). Afterwards adults return to the Western Baltic to overwinter in the sound between Denmark and Sweden (Øresund) (Nielsen et al. 2001) (Fig. 1). Note that our current knowledge on annual migration patterns of WBSS herring is predominantly based on intensive mark-and recapture studies in the early 1970s (Weber 1971; Biester 1979) and the use of “parasite tags” (e.g., the nematode Anisakis simplex serving as indicator of North Sea waters, Parmanne et al. 1994; Zander and Reimer 2002). Therefore, there is an urgent need to revisit this migration pattern and further explore the migration triggers (see "Processes influencing spawning biomass and spawning time" and "Current research needs and future research avenues to support management strategies" sections).
Previous studies suggest that recruitment success in WBSS herring is established by the time larvae reach a total length of 20 mm (Oeberst et al. 2009). Based on this finding, a recruitment index (the N20) was created based on the number of larvae reaching 20 mm by the end of the spawning season (i.e., June) during the Rügen Herring Larval Survey (RHLS) in Greifswald Bay. This N20 index seems to reflect well the recruitment dynamics of the whole WBSS stock and, thus, it has been used within the calculations of the recruitment index in the WBSS assessment (Oeberst et al. 2009; Polte et al. 2014; ICES 2021b). A second recruitment index, the GERAS age 1 index, is estimated based on the number of 1-yr-old juveniles observed during the German Autumn Acoustic survey (GERAS). The GERAS is part of an ICES-coordinated international program of surveys that provide annual information on stock size and structure of small pelagic fishes in the Baltic Sea. The GERAS covers the western Baltic Sea from the area connecting to the North Sea to the Arkona Sea (Fig. 1). The GERAS age 1 index is in good agreement with the N20 age 0 index (Fig. 2c) and the year-class strength estimates obtained from stock assessment (Oeberst et al. 2009).
WBSS herring recruitment has been continuously low since 2004 (Fig. 2). Current knowledge suggests that recruitment is impacted by a complex interaction of factors, including warming, eutrophication, and habitat degradation. These factors affect spawners, eggs and larvae in different ways. In the following we discuss how bottom-up and top-down processes impact each of these three life stages and what are their potential consequences for recruitment success of WBSS herring.
Processes influencing spawning biomass and spawning time
For most fish species and stocks, the relationship between the SSB and number of offspring is extremely variable and poorly explained by predictive models. This is especially true for small pelagic fishes (Cury et al. 2014). However, a minimum number of spawners is, in any case, needed to ensure a recruitment high enough to sustain the stock. For WBSS herring, the stock assessment and the corresponding specific reference points were re-evaluated in 2018 by ICES (ICES 2018a). The assessed SSB is now clearly below the biomass limit reference point (Blim of 120 000 t, Fig. 2B), defined as the threshold below which the recruitment must be assumed impaired due to an insufficient number of spawners (ICES 2021b). The fecundity and maturity of WBSS spawners is known to vary interannually (Gröhsler and Müller 2004) and most likely even differs between spawning cohorts within the same year. This variability is expected to have a profound effect on egg survival. For example, work on Baltic herring spawning in the Finnish Archipelago by Laine and Rajasilta (1999) reported that survival and hatching success of eggs was higher in females with better body condition and lipid profile. Female condition, in turn, depends on the feeding conditions experienced the previous summer (Rajasilta et al. 2019). Unfortunately, extensive studies on potential consequences of parental conditions for reproductive success are still lacking for WBSS herring. A low SSB alone may not explain the low recruitment of herring in the Western Baltic Sea during the last decade, but it probably magnifies the effects of unfavorable environmental conditions, particularly if fishery-induced demographic changes decrease the resilience of this stock to adverse environmental conditions.
WBSS herring are highly migratory fish, and particular environmental signals are probably involved in the initiation of spawning migrations. However, these aspects are not well studied yet. Some studies suggest that spawning is triggered by certain temperatures or light regimes. For example, the onset of spring spawning in Greifswald Bay seems to consistently start once temperature exceeds the threshold of approximately 4 °C (Klinkhardt 1986; Polte et al. 2021). The latter authors have shown that the first day of the year when this 4 °C threshold is reached in Greifswald Bay is occurring earlier every year, due to a higher frequency of mild winters that start later and last shorter. Ongoing work also suggest that earlier spawning is strongly related to warmer temperature during the previous autumn cooling period (N. Ory, C. Clemmesen, unpublished data). Both late winter onset and short duration seem to negatively impact WBSS herring reproductive success and larval and juvenile production (N20 age 0 and GERAS age 1 indices), potentially due to a temporal mismatch between exogenously feeding larvae and their suitable prey. For example, 1996 was an exceptionally good recruitment season, preceded by a long, cold winter (i.e., temperatures < 4 °C) that lasted for 4 months (Fig. 3). Hatching started in early May and feeding larvae only appeared in late May after the onset of the zooplankton bloom in this area. In contrast, 2020 had the lowest recruitment on record in the time series. This year was an example of a milder winter with a late winter onset (February) and a short duration (1 month). If hatching starts to occur in March, larvae may not be able to find enough prey in April (Fig. 3). This agrees with the observations that the contribution of the early spawning cohorts to the surviving year class is fading, simultaneously to the decrease of the overall recruitment strength (Polte et al. 2014). Given the potential consequences of this shift in the phenology of spawning and early larval feeding, further research on the topic is needed both regarding cues that initiate spawning and environmental controls on lower trophic level production driving potential match-mismatch between herring larvae and their prey (see "Processes influencing spawning biomass and spawning time" and "Bottom-up and top-down processes impacting embryonic stages" sections).
Conceptual figure showing the phenology of temperature, phyto- and zooplankton bloom and herring spawning during A a long, cold winter (e.g. season 1995/1996) and B a short, warmer winter (e.g. season 2019/2020), based on the dynamics described for Greifswald Bay (Sect. 3, Polte et al. 2021). The figure displays temperatures below 4 °C (blue), phytoplankton bloom (green), zooplankton bloom (yellow), presence of spawners (dark orange), yolk-sac larvae (light orange) and exogenously-feeding larvae (white) in the Bay. Presence of spawners is estimated from landings and observations from recreational fishers in the area (Polte et al. 2021). Presence of larvae is derived from the observations during the Rügen Herring Larval Survey. Note in warm years the plankton bloom starts earlier but the zooplankton bloom has a much lower magnitude
Bottom-up and top-down processes impacting embryonic stages
WBSS herring eggs are attached to benthic substrates such as macrophytes. This non-drifting embryonic life-stage minimizes the risk of individuals being passively dispersed into unfavorable habitats, but the fate of these embryos completely depends on the prevailing local environmental factors and local predation. Local environmental factors are probably more variable and potentially extreme in shallow coastal spawning areas, such as Greifswald Bay and Kiel Fjord, compared to deeper waters farther offshore. So far, the best studied bottom-up factors impacting embryo development and survival in WBSS herring are related to the physicochemical environment, such as eutrophication, habitat modification and temperature (Fig. 4, "Environmental factors" section). In terms of top-down factors, the interaction with the resident coastal fauna (i.e., predation) has also been addressed ("Predation" section).
Bottom-up and top-down factors influencing Western Baltic Spring-Spawning herring life cycle and recruitment. Line thickness indicates the number of studies (included in this review) that explored the corresponding driver. Line color indicates major knowledge gaps, with red being more important. Dashed lines indicate drivers not specifically studied for this herring population
Quantity and quality of spawning grounds
WBSS herring display high site fidelity for spawning grounds, where adequate spawning substrates are required (Kanstinger et al. 2018; von Nordheim et al. 2018). For this stock, spawning habitat degradation is a serious threat, either due to coastal modification (e.g., dredging activities) or eutrophication. Although Neb (1952) reported that WBSS herring used hard substrates for spawning in the Kiel Fjord, field observations on spawning behavior in Greifswald Bay showed a clear preference for vegetation (von Nordheim 2019). Macrophytes are probably the most important spawning substrate for WBSS herring, but their coverage has dramatically decreased in some areas of the western Baltic. For example, Kanstinger et al. (2018) estimated a reduction of 95% in the macrophyte coverage in Greifswald Bay in the past century caused by increased water turbidity linked to eutrophication (Munkes 2005). The macrophyte community is nowadays restricted to the littoral zone shallower than 3.5 m depth (Moll et al. 2018; Kanstinger et al. 2018). This spatial compression goes hand in hand with a reduced availability of the macrophyte species that herring could use as spawning substrates. In eutrophicated waters, certain algae, such as the epiphytic brown algae Pylaiella littoralis, can have mass occurrences in herring spawning areas, which can lead to massive egg mortalities up to 100% in Greifswald Bay (von Nordheim et al. 2020) and other Central Baltic spawning grounds (Aneer and Nellbring 1982). These negative effects on herring eggs are likely related to the release of algal biotoxic exudates (Aneer 1987), as has also been suggested for the red algae Furcellaria spp. in the eastern Baltic (Rajasilta et al. 1989, 2006; Rajasilta 1993). Besides macrophyte species, substrate structural complexity is also important. In a field experiment, von Nordheim et al. (2018) showed that mean egg mortality can be up to three times higher on substrates with lower compared to higher complexity. This effect was more pronounced at the end of the season when temperatures increased, highlighting the importance of accounting for interacting factors.
Due to increased eutrophication and the loss of vegetated spawning grounds, herring eggs are concentrated into smaller spatial aggregations in shallow spawning beds (up to 1.5 m depth). This can amplify the negative impact of other factors such as smothering due to oxygen depletion by multi-egg layering (see Sect. “Environmental factors”), increased predation pressure at high egg densities (see "Predation" section) and mechanical damage due to storms. Field experiments investigating the impact of storm events in Greifswald Bay reported that approximately one third of all eggs could be detached from the substrate in a shallow spawning bed and be partly washed ashore (Moll et al. 2018). Given current projections, the increased frequency of storm events (Coumou and Rahmstorf 2012) and eutrophication will remain major issues in the Baltic Sea (Andersen et al. 2017). Both factors will interact with others (e.g., macro algal blooms, reduced substrate complexity, multi-egg layering and increased predation pressure) likely leading to negative, synergistic effects impacting on herring egg survival in Baltic spawning areas in the future.
Environmental factors
Temperature
Temperature has direct and indirect effects on the pace of embryonic development of marine fishes (Peck et al. 2012a). WBSS herring eggs show highest hatching rates between 7 and 13 °C (ca. 7–12 days after fertilization), but eggs still display hatching rates > 50% between 5 and 19 °C (Peck et al. 2012b). This thermal tolerance is well within the temperature ranges herring eggs experience in Greifswald Bay and Kiel Canal during spring, and thus direct negative effects of temperature on egg survival and development are not expected (Dodson et al. 2019; Polte et al. 2021). However, temperature may have an important indirect impact on egg survival. For example, increased temperature leads to a shorter length-at-hatch which can impact larval survival through various mechanisms such as decreased yolk reserves and mouth gape (Busch 1996). Also, temperature can interact or modulate other factors, such as hypoxia sensitivity (see "Oxygen" section), or feeding rates and abundance of predators (see "Predation" section). Therefore, gaining further knowledge on the combined effects of temperature and other stressors is essential.
Oxygen
Shallow WBSS herring spawning grounds are usually well-oxygenated due to the wind-induced vertical mixing (Polte et al. 2014). However, hypoxic events can occur at small spatial or temporal scales due to eutrophication and habitat degradation, due to, for example, multi-egg layers on existing vegetation, thick algal mats or strong phytoplankton blooms. WBSS herring eggs seem to be able to tolerate hypoxic conditions (to some extent) for hours or days. Experimental work has shown that hatching success remained high for oxygen saturations down to 20%, although size-at-hatch was affected by oxygen level (Braum 1973). Eggs exposed to lower oxygen levels (15–20% oxygen saturation) showed embryo malformations, lack of heart contractions and no larval hatching (Braum 1973, 1985). Note these experiments were done at relatively cold temperatures (8 °C), so further research is needed to fully understand the combined stress of hypoxia and warming for the different egg cohorts throughout the spawning season.
Despite some capacity to deal with hypoxic conditions, large egg mortalities have been reported in relation to multi-egg layers or particle deposition on the surface of WBSS herring eggs. Several herring populations, including WBSS herring, have been observed to spawn their eggs in multiple layers due to a reduced availability of suitable spawning substrates (see "Quantity and quality of spawning grounds" section) and successive spawning cohorts re-using substrates (Parrish et al. 1959; Hempel and Schubert 1968; Messieh and Rosenthal 1989). Laboratory experiments in WBSS herring revealed that egg survival significantly decreased with increasing egg layering (Finke et al. 2022). Similarly, the deposition of organic particles on the egg surface can also lead to problems with surface gas exchange and smothering (Braum 1985; Klinkhardt 1986). The reported effect of mineral particles (e.g., sand particles resuspended by coastal modification, dredging operations, and/or wind-induced hydrodynamics) on herring embryos varies within the different studies. While Kiørboe et al. (1981) observed no changes in embryonic development in Baltic eggs exposed to different levels of silt (5–500 mg L−1), another study on Pacific herring (Clupea pallasii) reported lower hatching success and increased deformities at concentrations > 250 mg L−1 (Griffin et al. 2009).
pH
Ocean acidification is a global stressor to marine environments that can have deleterious effects especially on early stages of fish, for example during the gastrulation phase (Dahlke et al. 2017). Experimental work performed on herring eggs from the Kiel Fjord suggests that they are tolerant to elevated CO2 levels (~ 1000 µatm pCO2 expected to occur by the end of the century (RCP 8.5, IPCC 2019). But even elevated pCO2 levels (up to 4600 µatm) did not influence embryogenesis (fertilization success, embryonic developmental time, embryo malformation and mortality, hatching rate, length and weight and yolk area at hatch, otolith size) of herring eggs incubated at 14 °C (Franke and Clemmesen 2011). Additionally, the RNA:DNA ratio, a biochemical proxy for growth, of newly hatched larvae was also not influenced by elevated pCO2 up to 2900 µatm but decreased slightly at 4600 µatm. A follow-up study reported that spawning time modulates egg tolerance to pCO2 levels, as offspring originating from late spawning events (May) showed decreased fertilization success and increased mortality with increased pCO2 (380 to 4000 µatm) compared to offspring from mid-season spawners (April) (Bodenstein 2012). The author also did not find significant changes in the number and size of chloride cells (the ion regulatory organs in embryos until gills are developed) during embryogenesis up to 4000 µatm.
WBSS herring eggs seem to be more tolerant to high pCO2 than those from other herring populations. For example, Norwegian spring spawning herring showed increased malformation at hatch when reared at 1000 µatm (Leo et al. 2018), and mortality of Pacific herring eggs increased at 1200 µatm compared to those at ambient conditions but only when reared at warm temperatures (16 °C) (Villalobos et al. 2020). Franke and Clemmesen (2011) suggested that the high tolerance of WBSS eggs may be related to the high natural variability of pH in the Baltic Sea. In the Kiel Fjord, pH varies between 8.2 and 7.6 during the herring reproductive season in spring, and pCO2 levels > 2300 µatm have already been registered during late summer (Thomsen et al. 2010; Melzner et al. 2013; Reusch et al. 2018).
Chemical contaminants
Herring eggs in spawning beds close to urban centers with industrial areas and intensive farming are generally exposed to relatively high concentrations of pollutants and hazardous compounds (Reindl et al. 2015). Heavy metals, for example, can have a severe impact on fertilization, egg development and hatching success, depending on exposure time and concentration levels (Rosenthal and Alderdice 1976). In laboratory experiments with WBSS herring eggs, negative effects on egg survival were more pronounced for copper than for cadmium and lead (von Westernhagen et al. 1979). Larvae showed reduced embryo survival (< 10% viable hatch) at concentrations of 16.7 µg L−1, which is a lower threshold than that for other fish species (von Westernhagen 1988). Experiments with other pollutants, such as ferric hydroxide (“red mud”, a by-product in the aluminum production) and sulfuric compounds (a by-product of fossil fuel combustion), reported reduced WBSS herring egg survival (e.g. 50% survival at 1 ml L−1 ferric hydroxide; Rosenthal 1971), and the presence of malformations in newly hatched larvae at relatively low concentrations (e.g. body shrinkage and bending at dilutions 1:8000 of sulfuric pollutants; Kinne and Rosenthal 1967).
Numerous pollutants (e.g. herbicides, pesticides) are often highly persistent in the environment, accumulating in fat tissues of fish, such as in WBSS herring adults (Hansen et al. 1985; Karl and Ruoff 2007). These compounds are transferred to the gonads, which can reduce egg production up to 80% (von Westernhagen 1988) and larval survival (Schubert et al. 2016). Polychlorinated Biphenyls (PCBs) and other chlorinated hydrocarbons (such as Dichlorodiphenyltrichlorethane, DDT), were detected in the gonads of ripe and running WBSS herring females (Hansen et al. 1985). Although the PCB concentrations observed in the female gonads were variable (19–241 ng g−1), the majority of the samples was below the assumed threshold for acute toxicity and mortality (< 120 ng g−1) (Hansen et al. 1985). However, note that the levels of organochlorine concentrations (DDTs, PCBs) seem to be strongly correlated with age and lipid content (Perttilä et al. 1982), indicating a negative impact on older herring females that generally display higher fecundity (Kändler and Dutt 1958).
Chemical warfare agents can be another important source of pollution near specific dumping sites in the Baltic Sea, such those in the Bornholm and Gotland Basins. These areas have high genotoxicity risk due to the presence of genotoxic agents (e.g. sulfur mustard or arsenic-containing compounds) (Baršienė et al. 2014). The authors reported higher environmental geno- and cytotoxicity levels near the dumping sites, although their ultimate effect on WBSS herring reproduction success remains unknown.
The impact of oil spills has not specifically been studied for WBSS herring, but studies on Pacific herring and other Baltic herring populations have shown devastating effects of oil exposure on developing eggs. Intensive research after the spills from “Exxon Valdez” in 1989 and the “Cosco Busan” in 2007 on the western coast of the USA reported high mortality rates, significant deformities, and cytogenetic abnormalities in Pacific herring eggs and larvae (e.g. Incardona et al. 2012 and references herein). In the Baltic Sea, reduced hatching success (50%) and high number of larval malformations has been observed in areas affected by oil spills (e.g. “Tsesis” in 1977 and “M/T Antonio Gramsci” in 1987) compared to similar areas without oil pollution (Aneer and Nellbring 1982; Rahikainen et al. 2017). These results are in line with a laboratory study revealing that most Baltic herring larvae exposed to crude oil constitutes died or displayed malformations (Lindén 1978). Considering the steady increase in marine shipping traffic in the Baltic Sea (HELCOM 2018), oil spills pose an immense risk to the marine environment and can have severe impact on the successful reproduction of ecologically and economically important fish species, such as herring. In a recent, global review of research conducted on small pelagic fishes since the new millennium, the direct effect of pollution was one of the least studied factors (Peck et al. 2021).
Predation
Distinct herring groups use a broad variety of different coastal ecosystems for spawning, which leads to differences in potential spawn predators. For example, Pacific herring spawns preferably on intertidal spawning beds along the North American coast. Their eggs are primarily preyed on by gulls and other marine birds (Bishop and Green 2001; Lok et al. 2008), but also by terrestrial predators visiting the shoreline during low tide. In contrast, WBSS herring eggs are mainly spawned on permanently submerged beds, being out of reach for non-diving birds or terrestrial predators. Consequently, fishes and diving ducks are the main predators of WBSS herring eggs (Tibbo et al. 1963; Richardson et al. 2011; Stempniewicz 1995). Several investigations in the Baltic Sea (including Greifswald Bay) have supported the assumption that diving water fowls, such as the long-tailed duck Clanguela hyemalis, switch from their usual prey (molluscs) to herring eggs in spring (Leipe 1985; Stempniewicz 1995) and aggregate on known spawning beds for feeding (Žydelis and Esler 2005). Since long-tailed duck populations are at a long-term low in the Baltic, this species no longer represents a major threat to herring eggs.
Resident fishes have been observed to feed on herring eggs (Scabell 1988; Rajasilta 1993), but attempts to quantify the impact of those predators on the overall early life survival are extremely rare (Kotterba 2015). In Greifswald Bay, the three-spined stickleback (Gasterosteus aculeatus) represents a dominant fish species during the herring spawning time. Combining laboratory and field experiments, Kotterba et al. (2014, 2017a) demonstrated that sticklebacks have the potential to consume a substantial amount of herring eggs, while other predators such as river perch (Perca fluviatilis) were of minor importance. At the beginning of the spawning season, when predator numbers are still very low and herring egg concentrations extremely high, the predation impact on egg survival was negligible. However, later in the season (i.e., May) egg mortality due to predation could rise to 40%. Predation experiments showed a fourfold increase in the daily herring egg consumption by sticklebacks at 15 °C compared to 11 °C (Kotterba et al. 2014). Another important aspect is that the egg predation rate was higher when the initial number of eggs offered to the predators was high, suggesting that habitat compression and fragmentation of macrophyte beds may also trigger predation rates. These patterns are particularly interesting because interannual recruitment variability in recent years seems to be related to the fate of the larvae hatched in the second half of the spawning season (when predation is higher) (Polte et al. 2014). Altogether, these findings further highlight that an amalgam of interacting stressors (i.e., predation rate, temperature, habitat destruction) combine to influence early life stages survival and recruitment success.
As in other estuarine systems, the fish community within the Baltic Sea underlies a strong seasonality (Elliot and Hemingway 2008). The observed shifts in the phenology of WBSS herring spawning time (Fig. 3) may thus lead to rather unpredictable changes in the community and abundance of egg predators. This is especially true for the current invasion of diverse non-indigenous species in the Baltic Sea. One example is the round goby (Neogobius melanostomus) that has become very abundant during the last decade in many different Baltic habitats including herring spawning beds. Juvenile individuals of this species have been observed feeding on herring eggs (Wiegleb et al. 2018) and, assuming a further spread of this species, might represent a future threat to herring reproduction. So far, it cannot be predicted how the introduction of non-indigenous species, climate change and other ongoing, substantial alterations of the coastal ecosystems might influence top-down mechanisms that regulate herring egg survival.
Bottom-up and top-down processes impacting larval stages
Prey abundance and type
The spatio-temporal match with food of adequate type and quality (e.g. size, species, nutritional value) and quantity is a prerequisite for the survival of early life stages of marine fishes. After hatch (typically around 6–7 mm standard length, Peck et al. 2012a), WBSS herring larvae fuel their metabolism exclusively with energy from their yolk sac, which lasts for about 70 degree-days (e.g., 7 days at 10 °C, Illing et al. 2015). As the yolk sac is consumed, the esophagus progressively opens, enabling the larvae to feed externally (Busch 1996). At the onset of first feeding (around 8.5 mm larval length at 10 °C, Illing et al. 2015), esophagus diameter (rather than maximal mouth gape) limits the prey size larvae can ingest. At this stage, ingested particles can be as small as 5 µm (nanoplankton fraction), although larger particles (77–104 µm, microplankton fraction) are preferred. Later, larvae of 8–10 mm in length selectively feed on metazoan microzooplankton: copepod nauplii, mostly Acartia spp. (80–87% of the stomach contents in Greifswald Bay), and rotifers from Synchaeta spp. (Busch 1996; Hesse 2010).
The range of preferred prey size of WBSS herring larvae is positively correlated with mouth gape size through ontogeny. While esophagus diameter is the major limitation to prey size for early larval stages, prey escape behavior (e.g., copepod jumping) becomes more important in later larval stages. Therefore, early larvae typically forage on prey that is about 60% in length of the mouth gape opening (Pepin and Penney 1997; Hufnagl and Peck 2011). As larvae grow, they add various other items to their diet, such as Acartia spp. and Eurytemora affinis copepodites, cladocerans, cirriped nauplii, and gastropod and bivalve veliger larvae (Spittler et al. 1990; Busch 1996; Hesse 2010; Lindegren et al. 2011; Arula et al. 2012; Paulsen et al. 2016). Studies on herring populations elsewhere suggest that larvae can be very selective feeders targeting certain copepod species and stages (Checkley 1982; Pepin and Penney 1997; Robert et al. 2014). Yet field studies in Greifswald Bay and Kiel Canal suggest more general foraging patterns and consumption of the most abundant species in the nursery habitat (Paulsen et al. 2014b, 2016 and references herein). These studies suggest that WBSS herring larval growth in Kiel Canal and Greifswald Bay is more strongly related to high density of prey, but that high food quality (DHA fatty acids) can compensate for low food quantity and vice versa, at least during the two studied springs (Paulsen et al. 2014a,b).
Copepods and other metazoans have been the focus of most diet studies in larval fishes, but protists, such as ciliates and dinoflagellates, can play an important role in many species, including herring (Montagnes et al. 2010 and references herein). For example, protists can upgrade the trophic level of copepod nauplii (Veloza et al. 2006). Laboratory experiments with first-feeding WBSS herring larvae have shown that matching phytoplankton, protists and copepod nauplii during spring increases the “window of opportunity” for larvae to switch from yolk-sac to external feeding (Illing et al. 2015). Although modelling studies suggest that protists alone cannot sustain larval herring growth over the long term (Bils et al. 2017), protists can complement metazoans in the diet and improve the nutritional quality of consumed prey.
Current changes in the spawning phenology of WBSS herring have cascading effects, influencing match-mismatch dynamics of larvae and their prey (Fig. 3). As previously explained, mild winters have been more frequent in recent years, causing an earlier spawning and thus an earlier presence of feeding larvae in the system. These larvae may find themselves not matching their planktonic prey, as the production (and succession) of the zooplankton bloom is not only driven by temperature, but also by phytoplankton abundance and day length among others (Diekmann et al. 2012). This mismatch has negative consequences for larval growth, condition and survival, as has been shown for herring in the Gulf of Riga (Arula et al. 2016). For example, feeding onset in WBSS herring was delayed by up to 4 days at low compared to high prey concentrations (7.5 vs. 120 copepod nauplii L−1) (Kiørboe et al. 1985). Subsequent laboratory experiments indicated that feeding rate and growth rate is limited at densities < 30–50 nauplii L−1 (Munk and Kiørboe 1985; Kiørboe and Munk 1987). More recent laboratory experiments have shown that WBSS herring larvae have developed mechanisms to cope with prey-poor environments (e.g. lowering their standard metabolic rate and swimming activity (Illing et al. 2018; Moyano et al. 2018), but they will need to find suitable prey before 100 degree-days when they will reach their “point of no return” (Blaxter and Hempel 1963; Illing et al. 2018). Long-term datasets on prey type and abundance through the spawning season in Greifswald Bay and Kiel Canal are not available at the moment but there is work in progress (L. Livdane, C. Clemmesen, pers. comm.). These datasets will be valuable to test the importance of starvation-driven mortality during the larval stages and its variability within and across years. Previous short-term studies suggested that the Kiel Canal had higher prey abundance associated with faster larval growth rates in early spring (up to late May) compared to larvae in Greifswald Bay, which had better prey fields later in the season (Peschutter 2008; Paulsen et al. 2014b). This would underpin observations of higher survival in late rather than early larval cohorts in Greifswald Bay (Polte et al. 2014), despite increased egg predation rates and increased metabolic demands due to the warmer temperatures later in the spring (Kotterba et al. 2017a; Moyano et al. 2020).
Environmental factors
Temperature
Within certain limits, warmer temperatures support more rapid rates of growth and development, and result in larvae reaching faster important morphological landmarks (e.g., notochord flexion, increased myotome height) associated with increased swimming performance, supporting more efficient foraging, and the capacity to escape predators and avoid passive transport into unfavorable areas (Moyano et al. 2016). However, warmer temperatures also imply increased metabolic needs and, thus, higher prey densities are required to support growth (Allan et al. 2022).
A recent statistical analysis of larval herring abundance and distribution in Greifswald Bay reported the highest abundance of yolk-sac larvae within an optimal thermal window of 9 to 13 °C (Dodson et al. 2019). The authors point out that this window has exhibited a major temporal contraction between 1995 and 2000, which may have contributed to the lower herring recruitment observed after 2000. Highest abundances of preflexion and postflexion larvae were observed between 10 and 13 °C and between 14 and 19 °C, respectively. Parallel laboratory experiments reported cardiac problems (arrhythmia) already at 19 °C, and loss of equilibrium (critical thermal maximum, CTmax) appeared at 21 °C (Moyano et al. 2017, 2020). These high temperatures have been rarely registered in Greifswald Bay and Kiel Canal, and are, thus, unlikely to impact larval survival in nature. However, temperatures exceeding the optimal thermal range can have a large impact on larval growth and survival, as observed in herring larvae in the Gulf of Riga (Arula et al. 2015). Based on cardiac and growth estimates, 16 °C has been identified as an optimum temperature for herring larvae obtained from strip spawning Greifswald Bay adults (Moyano et al. 2020). These authors reported that the number of days above this 16 °C threshold have significantly increased in this area in recent years ("Processes influencing spawning biomass and spawning time" section), and used this variable to create a thermal threshold index. This physiological indicator has been related to the drop of the N20 age 0 and GERAS age 1 recruitment indices. Taken together, the temporal match between larvae and warmer water temperature and plankton blooms seems to be of high importance for rapid growth and development, allowing larvae to reach a state of competent swimming which supports foraging, selecting favorable habitats, and reduces predation risk.
Other environmental factors
Most studies exploring effects of environmental factors other than temperature in WBSS herring have focused on the egg stages rather than larvae. For example, no experiments have yet investigated the effect of acidification on WBSS herring exogenously feeding larvae. Previous work on Norwegian spring spawning herring larvae (up to 40 days post-hatch) reported negative impacts above 1800 µatm pCO2 on growth, development, condition, and tissue formation (Frommel et al. 2014), although other traits such as swimming behaviour and proteome structure were unaffected in fish from the same experiment (Maneja et al. 2015). At lower pCO2 (900 µatm), larvae were not impacted by increased pCO2 (Sswat et al. 2018b), and in fact, larval survival increased 19% at high pCO2 (760 µatm) during an in-situ mesocosm study due to increased prey abundance at those conditions (ambient temperature from 8 to 15 °C, Sswat et al. 2018a). While the conditions in the WBSS herring nursery grounds have a highly variable pH that would in principle lead to a higher tolerance (see Sect. “pH”), the combination with temperatures above 16 °C would need to be assessed in the future, considering both the direct and indirect (food mediated) effects.
Previous studies on salinity tolerance of herring larvae from several North and Baltic Sea populations, including WBSS herring, suggest a hard-wired salinity threshold of 1.9 to 2.7, which increased with body size (Illing et al. 2016). Although salinity projections from that study suggest that Greifswald Bay and Kiel Canal will likely not experience salinities < 2 by the end of the century, freshening is already posing serious problems to other Baltic populations via direct effects on the fish (increased energy needed for osmoregulation) or via changes in the prey community (e.g., Bothnian Bay in Finland, Rajasilta et al. 2019). Such salinity-induced changes in the prey community may be relevant to larval growth and survival as the copepod production in the Baltic is strongly affected by their salinity tolerance (e.g. Temora longicornis vs Eurytemora affinis (Diekmann et al. 2012).
Predation
Predation is arguably the primary mortality driver for larval fishes (Bailey and Houde 1989; Fuiman and Magurran 1994). For populations of small pelagic fishes, predation on their larval stages can play a critical role and have implications for recruitment dynamics (Engelhard et al. 2014). For example, opportunistic feeding of Atlantic mackerel (Scomber scombrus) on Atlantic herring larvae has been observed in the Norwegian Sea at rates large enough to regulate year class strength (Skaret et al. 2015; Garcia et al. 2020). In the Baltic Sea, however, predation mortality of herring larvae in their coastal habitats seems to be mostly related to the temporal match-mismatch dynamics with gelatinous rather than piscine predators (Kotterba et al. 2017b).
Similar to herring eggs, fishes consuming Baltic herring larvae are most commonly the three-spined stickleback and river perch (Kotterba 2015). However, stomach analysis of both species suggest that they ingest negligible amounts of larvae (Kotterba et al. 2014, 2017b), as opposed to eggs (see Sect. “Predation”). Filial cannibalism, another potential cause for predation mortality of fish larvae, seems to be of minor importance in Greifswald Bay (Kotterba 2015). Altogether, these findings highlight that the transitional waters of Greifswald Bay seem to provide sufficient shelter from predation to WBSS herring larvae, as gelatinous predators (e.g., moon jellyfish Aurelia aurita and the non-indigenous ctenophore Mnemiopsis leidyi) appear later in the system (Kotterba et al. 2017b). On the contrary, A. aurita co-occurs with larval herring in late spring on other WBSS spawning grounds (e.g., Kiel Bight and Kiel Fjord), and can significantly reduce larval herring abundances by efficiently utilizing dense prey patches (Möller 1984; Schneider and Behrends 1998; Titelman and Hansson 2006). M. leidyi and the hydromedusa Sarsia tubulosa have also been observed in recent years during the entire herring larval season in the Kiel Fjord, sometimes at very high densities (C. Clemmesen, F. Mittermayer unpublished data). Ongoing work suggests that both species can potentially prey on herring larva, even in the presence of other prey (I. Stoltenberg, F. Mittermayer, unpublished data). With predicted warmer winters and a potential shift to jellyfish-dominated food webs, the role of jellyfish predators may become increasingly important for the recruitment dynamics of WBSS herring due to the competition for copepods and the direct predation on larval herring (Möller 1984; Ramirez-Romero et al. 2018).
From single factors to complex interactions and cumulative effects
The present review highlights the breadth of knowledge on WBSS herring recruitment gained in the last decade. Since 2011, 40 studies (out of the 68 specifically on WBSS herring cited here) have been published on WBSS herring early life (31 studies) or later stages (9 studies). Most of these studies conducted field sampling / experiments (48%) or laboratory experiments (28%). This research stresses that no single, dominant driver is responsible for the recent low recruitment in WBSS herring but that several interacting bottom-up and top-down factors affect each life stage differently. For example, the combination of warming and habitat degradation is leading to an earlier spawning in a restricted habitat, which magnifies the negative effects of storms, chemical contamination, predation or mass algal blooms on egg development and hatching success. However, our understanding of the extent of these interactions is still limited, hampering our ability to quantify their cumulative effect on herring recruitment. Most experimental studies have so far focused on single factors (e.g. temperature, spawning substrate type), although complex and unexpected interactions may occur between two or multiple stressors (Catalán et al. 2019). Further experimental work is thus needed to elucidate the magnitude of these interactions. Such information could be then applied to parameterize numerical models, which can be used to generate and test hypotheses to identify the most influential factors and/or most uncertain processes in field data (Ihde and Townsend 2017). So far these models, both statistical and mechanistic, only constituted 11 of all 68 studies on WBSS herring cited here, but advancing these tools will be the next logical step for future research effort.
One widely applied method to examine the possible influence of environmental factors on recruitment is including them in stock-recruitment (SSB-R) models to generate hypotheses about major bottom-up and top-down processes (e.g. Subbey et al. 2014; Szuwalski et al. 2015; Akimova et al. 2016b). Previous studies demonstrated a substantial improvement of stock-environment-recruitment models over conventional stock-recruitment relationships in WBSS herring. Cardinale et al. (2009) and Gröger et al. (2014) reported a significant influence of the Baltic Sea Index (BSI) on the goodness-of-fit of the SSB-R relationships in this stock. The BSI was defined as the difference in the sea level pressure anomalies between Oslo in Norway and Szczecin in Poland. It was introduced to characterize the dominant water circulation pattern (negative phase—outflow, positive phase—inflow) over the Skagerrak/Kattegat and the western Baltic Sea (Lehmann et al. 2002). In a later study, Péchuchet et al. (2015) identified nitrate concentration, water currents and salinity as key explanatory variables able to explain 80% of the residual variance in the SSB-R relationships in WBSS herring. The total zooplankton biomass and temperature were not significant factors in that study.
SSB-R models have been extensively used to identify the impact of relevant physical and biological variables. They mainly rely on correlations between environmental and stock parameters, and seldom can pinpoint an exact mechanism behind those correlations. Also, currently used statistical models struggle to include non-linearities in the form of thresholds and thus they do not perform well in the event of drastic changes in environmental conditions and/or regime shifts (unless these are incorporated in the model formulations as in Casini et al. 2010). Therefore, decision-making processes require a more mechanistic understanding of the relevant top-down and bottom-up drivers and a tool for testing scenarios to ensure future sustainable exploitation (Karp et al. 2019). The emergence of biophysical individual-based models (IBMs) in fish ecology in the 1990’s was a pivotal development in recruitment research. Highly flexible IBMs are well-suited to explore how individuals respond to the biotic and abiotic environment and how factors and processes interact impacting population dynamics. These IBMs have been a popular tool applied to generate and/or test hypotheses on key processes such as transport (retention or advection), starvation (prey availability) and/or predation (Werner et al. 2001; Peck and Hufnagl 2012). Some of the first IBMs were developed for Atlantic herring and other small pelagic fishes because of the strong responses of these species to changes in bottom-up (physical and biogeochemical) forcing. Those IBMs used a great variety of approaches to simulate fish early-life stages, e.g. a lagrangian particle tracking approach combined with temperature-dependent growth and mortality (e.g. Akimova et al. 2016a, b), foraging bioenergetic models of fish larvae (e.g. Daewel et al. 2011), dynamic energy budget (DEB) models for the full life cycle (e.g. Bueno-Pardo et al., 2020), and models of active behaviour and migration (e.g. Huse and Fiksen 2010). More recently, IBMs have been created to explore how simultaneous changes in abiotic and biotic factors affect larval survival (e.g., temperature × transport × prey (Hinrichsen et al. 2012), transport × predation (Akimova et al. 2019)). Several IBMs have been constructed for Atlantic herring. For example, a seminal modeling study by Bartsch et al. (1989) explored the hydrodynamic transport of herring larvae in the North Sea. Hufnagl et al. (2015) and Fiksen and Folkvord (1999) used larval bioenergetic models to study larval starvation and growth in the North Sea and on the Norwegian shelf, respectively.
In the western Baltic Sea, Bauer et al. (2013, 2014) used particle backtracking to infer strong retention of young herring larvae and spawning site selection in Greifswald Bay. Hufnagl and Peck (2011) applied a 1D larval physiological model to investigate the suitable spawning times of herring populations in the North Atlantic, including WBSS herring. Later this model was combined with laboratory experiments to investigate the role of behavioral and metabolic adaptation of WBSS herring larvae to suboptimal feeding conditions (Illing et al. 2018). However, no model has attempted to simulate in situ foraging success and survival of WBSS herring larvae. Given recent advances in larval herring IBMs and a large number of observational studies (e.g. phenological shifts in spawning time, prey and predator fields), applying mechanistic models appears to be the next, logical step to study cumulative impacts on the recruitment success of WBSS herring.
Current research needs and future research avenues to support management strategies
Despite the significant advances in knowledge reported above on the ecology of WBSS herring and the potential drivers impacting the recruitment process, it is still difficult to precisely identify where the bottleneck lies. Furthermore, from a management standpoint, it remains challenging to translate the outcomes of existing research into a clear, unified set of equations/indicators useful for stock assessment and advice. However, we have clear indications on priorities for further work. In the following, we have summarized which avenues future WBSS herring research should address and, specifically, the work that would be most impactful and relevant to support the sustainable management of this stock (Table 1).
WBSS herring is currently managed using a single area, single species and single spawning component assessment model (ICES 2021b). Similar to most other stock assessments, the model for WBSS herring does not incorporate any environmental or ecosystem processes beyond the variable weight-at-age relationships. Since the last benchmark, the model has been extended to include exploitation by multiple fishing fleets attached to different areas (ICES 2018a). While this has helped to support the complications of a scientific advice across multiple fishery management areas and capture some aspects of the seasonal availability of the stock to the different fisheries, it did not explicitly address the complexity of spatial use in WBSS herring and associated population mixing described above. In this sense, there are two major critical knowledge gaps that should be prioritized to provide science-based advice to management: i) research on migration pathways and ii) meta-population structure (Table 1).
Migration is a strong life history trait in WBSS herring and it is plausible that population variability and productivity is linked to one or more of the many aspects related to migration, such as changes in energy allocation to reproduction and growth as suggested for other herring stocks (dos Santos et al. 2021). WBSS herring surveys are static in seasonal coverage (Fig. 1) and thus they probably fail to capture changes in migration pathways. Our current knowledge on migration routes in WBSS herring relies on telemetry data from the 1970s and most likely does not reflect the present stock situation and migration patterns ("Life cycle and recruitment process" section). For example, the GERAS has since 2016 identified distinctly lower abundances of herring in the Øresund (overwintering ground) during autumn. While the survey indices are in line with other data sources indicating a decline in spawning adult abundance, it is unclear whether a temporal and/or spatial shift of the overwintering migration or habitat use might have occurred. Revisiting migration routes, their timing and the environmental cues that trigger them is therefore urgent. This knowledge could be directly used in the assessment process, for example, to improve survey-based estimates of abundance or to generate environmentally-based estimates of overwintering areas that are later used to modify the spatial coverage of the GERAS survey and commercial sampling each year, as it is done with the spawning habitat of Pacific sardine (Sardinops sagax) in the California Current during the spring acoustic surveys (Zwolinski et al. 2011). Recent technological advances, such as the use of stationary, autonomous echosounders in key positions (e.g., Greifswald Bay, Øresund) could help in identifying and quantifying migration movements. Using modern telemetry techniques, one could explore the inter-individual responses to environmental cues and how these responses relate to the different spawning waves observed in Greifswald Bay. In this sense, it is still unknown whether the spawners belonging to the same wave have the same age, size-class or condition, and the potential consequences this could have for offspring fitness (Huang et al. 2022). Also, do all spawners come back to the same spawning grounds where they were born? Homing to natal spawning area was observed to be relatively high (70%) in Greifswald Bay using otolith microchemistry (Moll 2018), but further information expanding on different spawning areas is needed.
Gaining a holistic understanding of population structure, demographics, and associated temporal fluctuations is the second key aspect that requires further research. WBSS herring is considered a meta-population and, as such, its productivity depends on the relative, dynamic contribution of the different spawning components. The productivity of these components may, in fact, change in response to different drivers, providing a higher resilience towards changing conditions to the overall metapopulation. For example, autumn spawners were a dominant fishery target in the Baltic Sea until the late 1960s (Weber 1971), but their abundance drastically declined in subsequent decades. Recurrent sampling of large, post-flexion larvae (> 30 mm) during late winter in Greifswald Bay, confirmed as autumn-spawned larvae by otolith age reading (Janke 2019), indicated that autumn herring still reproduce in the Western Baltic Sea. This raises the question of whether the current environmental conditions would favor autumn- over spring-spawning for WBSS herring and under which mechanism(s) such a shift in phenology would occur. These potential shifts in seasonality and productivity call for a detailed monitoring of the different spawning components as currently done for other stocks (e.g. North Sea Autumn Spawning herring).
Another essential aspect that deserves further attention is assessing the level of mixing with neighboring herring stocks (i.e. Central Baltic Spring Spawners, North Sea Autumn Spawners, Norwegian Spring Spawners). Molecular methods have been used to obtain snapshots of this mixing in certain regions in the Skagerrak, Kattegat, and western Baltic (Bekkevold et al. 2015). Since these components regularly mix in commercial fish catches and fisheries-independent observations, the development and validation of indicators based on simple metrics routinely collected (e.g. age, length) would be very useful to support management. For example, increasing numbers of Central Baltic herring have been migrating into the western Baltic in recent years. A stock separation function to distinguish WBSS and Central Baltic herring based on growth parameters has been developed (Gröhsler et al. 2013) and applied in the years 2005–2020 in the GERAS survey (Schaber and Gröhsler 2021), which is routinely used as tuning index in the WBSS herring assessment (ICES 2021b). This method still needs to be validated (e.g., genetics, otolith microchemistry) and the temporal stability of mixing assessed before it could be routinely implemented to partition the commercial catches in the area by stock (ICES 2018b). Overall, increasing our understanding of spawning migration cues and meta-population structure and demographics would likely help disentangle factors leading to the current low SSB levels. It is worth noting that one of the challenges of WBSS herring compared to the neighboring herring stocks is that the relatively short time series available (starting in 1992) that has only recorded a steady decrease in recruitment and SSB, making it difficult to speculate about reference levels representative of a good status of the stock. Moreover, we want to emphasize the importance of the WBSS herring fishery closure under the current conditions to avoid potential issues with hyperstability (CPUE remains high although population size declines), as has been observed in North Sea herring in the 1970s (Bjørndal 1989). Although hyperstability would not directly impact the assessment, which is mainly based on fisheries-independent data, it would still impact the management of the stock as the fishery would be able to maintain profitability despite overexploiting the population.
Besides increasing our knowledge on migration routes and population demographics, there are other aspects related to the ecology, basic biology and/or physiology of WBSS herring that will be relevant for effective conservation and management of this stock. These aspects comprise:
-
Gaining a more profound understanding of life-stage specific physiological limits and plasticity to anticipate the effects of climate variability and change in WBSS herring productivity. Warming, oxygen limitation linked to eutrophication, chemical contamination and many other environmental factors discussed in sections "Processes influencing spawning biomass and spawning time" to "Bottom-up and top-down processes impacting larval stages" can seriously threaten WBSS herring productivity through reducing the survival of specific life stages, and the potential interaction of these stressors is still unknown for many commercially important fish and shellfish (Catalán et al. 2019). Experiments exploring the mechanisms behind these processes can be used to define physiological thresholds and create useful indicators for management and conservation purposes (e.g., Moyano et al. 2020; Karp et al. 2019). For example, species distribution models based on historical spatial observations for Pacific sardine and anchovy (Engraulis mordax) in the California Current displayed lower predictability skill under heatwave scenarios outside the observed temperature range in the past, underscoring the need for integrating physiological limits in these models to make them more robust towards a rapidly changing environment (Muhling et al. 2020).
-
Characterizing larval prey fields in the spawning/nursery grounds. Research is underway to explore prey fields in Greifswald Bay and Kiel Canal over several years and match them with observed larval survival and growth estimates (C. Clemmesen, L. Livdane, unpublished data). This information will allow investigating how potential food-limitation during the entire spawning season affects in situ larval abundances in both nursery grounds, and, ultimately, recruitment indices (N20 age 0 and GERAS age 0). Such analysis would specifically support our understanding of density-dependent processes, such as local prey depletion, and intra-specific competition that are likely important but understudied in most small pelagic fishes (Peck et al. 2021) and allow to assess a cumulative effect of the warming and prey-field changes observed in recent decades in these nursery areas. For example, if prey abundance displays a clear relationship with larval production (e.g. N20), it could be used directly as an indicator in the assessment models as has been done in other herring stocks (e.g. Gulf of Riga herring, Arula et al. 2016; ICES 2021a) or as part of a more holistic, integrative approach incorporating multiple factors affecting the recruitment process, from spawners to larval stages (e.g. Brosset et al. 2020).
-
Incorporating mesocosm experiments as a powerful tool to explore the direct and indirect (via food web) impacts of multiple stressors. Such mesocosm experiments have been successfully used in other herring populations to demonstrate, for example, that beneficial indirect effects via increased prey abundance can compensate for the limited negative direct effects of acidification in Norwegian spring-spawning herring (Sswat et al. 2018a).
-
Reevaluating the impact of top-down effects on egg and larval mortality (i.e., predation, intraspecific competition). Some of this work is already ongoing to evaluate the current phenology changes of different jellyfish species that now coexist with WBSS larval herring in the nursery grounds (F. Mittermayer, C. Clemmesen, unpublished data). Once the key players in predation mortality have been identified, their abundance could be integrated as a mortality term in the assessment models as it is done for other herring stocks (e.g. Atlantic cod, Gadus morhua, predation on Central Baltic herring, Skern-Mauritzen et al. 2016; ICES 2019, 2021a).
The mechanistic understanding of the recruitment process gained through research proposed in this section has important value in the stock assessment for WBSS herring as currently done, and also to incorporate considerations towards ecosystem-based fisheries management (Table 1). First, this information can have an important direct value for calculating the biological reference points later used in the assessment models, as understanding the drivers of productivity is necessary to evaluate the status of the stock and to provide scientific advice to governmental bodies. Second, we hope that such mechanistic information (e.g., physiological indicators) can be directly implemented in the assessment and forecasting to help ensure population diversity and resilience are realized in management actions. Although one may think that reliable climate forecasting may hinder the application of some of these physiological indicators in single-species assessment, seasonal forecasting has been successfully used to provide advice at operational time-scales relevant to specific fisheries (e.g., Hobday et al. 2018).
Management of WBSS herring is not trivial as the stock is of a metapopulation with a high spatial heterogeneity, returning to specific spawning grounds in shallow coastal areas but using more offshore areas to feed and overwinter, across national boundaries and fisheries management areas. Thus, an effective management of this population requires a close collaboration between ICES-coordinated management efforts and coastal zone management plans. WBSS herring spawning grounds have been reduced and fragmented due to anthropogenic stressors such as eutrophication and coastal modifications (e.g., construction of harbors). However, a proper evaluation of such habitat fragmentation, as well as its consequences for fish use and successful reproductive output, is lacking. Therefore, it is essential to carefully assess potential impacts of future coastal activities on these spawning grounds and establish monitoring programs to evaluate how habitat use by fish may have changed (Rosenthal et al. 1995). Future projects are essential to build a bridge between coastal planning and fisheries management. We want to emphasize the importance of developing frameworks that allow an integration of our current understanding of the ecosystem functioning at different geographical scales. There are already examples for species with complex life cycles, such as Atlantic salmon (Salmo salar), in which current stock management measures are integrated from local rivers to regional, national and ocean basin scales (Bull et al. 2022). Such a tiered management system will require new levels of cooperation at the regional, national and international levels, which could also advance the implementation of more ecosystem-based fisheries management considerations. Additionally, these latter authors describe how the assessment process for Atlantic salmon will soon implement a life cycle approach, which should provide more opportunities to integrate specific parameters to the different life stages and follow the multi-step approach to study the recruitment process (Hare 2014; Brosset et al. 2020).
Lessons learned from WBSS herring recruitment research
Coastal ecosystems are particularly exposed and sensitive to climate change and anthropogenic pressure (Reusch et al. 2018). The present review exemplifies the complexity in which these stressors (e.g., habitat degradation, warming, eutrophication) interact and differently impact each life stage of a small pelagic within a relatively simple food web. Such complex interactions have also been observed in stocks of other coastal small pelagic fishes. For example, the decline in productivity of Scotian Shelf-Bay of Fundy herring was associated with warming, decreased predation (leading to density-dependent competition during the larval stages) and increased fishing (Boyce et al. 2021), whereas prey mismatch and temperature have been suggested as major drivers of recruitment in the Gulf of Riga herring (Arula et al. 2015). The complex nature of the interactions among drivers (e.g., direct vs indirect effects of warming) calls for mechanistic approaches to inform models to help understand and disentangle these effects. Using mechanistic approaches is particularly important because the most dominant drivers may be non-stationary and change over time. For example, the impact of mackerel predation is now considered a major driver of recruitment success in Norwegian spring spawning herring, as mackerel continues expanding its distribution to higher latitudes due to ocean warming (Garcia et al. 2020). In this sense, we believe that the holistic approach (laboratory, field, modelling efforts) used in the last decade to investigate bottom-up and top-down factors impacting WBSS herring recruitment is the first step to generate knowledge and tools that support the challenges ahead while developing a more integrative, ecosystem-based fisheries management and promoting conservation of the stock.
Some of the identified knowledge gaps proposed here for WBSS herring exist for most small pelagic fish populations (Peck et al. 2021). For example, the need to further study the role of density-dependence in (meta-)population dynamics, the development of further mechanistic model approaches (to complement correlative statistics) to explore the influence of climate variability, and the use of mesocosm experiments were general recommendations as future avenues of research in small pelagic (Clupeid) fishes. However, WBSS herring research is already addressing many of these general recommendations, such as using a holistic approach to advance our understanding of bottom-up drivers, the sampling of juveniles and adults during the GERAS, and the use of otolith microchemistry to ascertain migration patterns. These points underscore that WBSS herring constitutes a well-studied stock, where the existing knowledge and holistic approach, together with existing knowledge gaps, can provide a powerful example when developing programs to study population productivity and recruitment in other fish stocks. Moreover, given the severe anthropogenic impacts in the Baltic Sea compared to other coastal areas, WBSS herring could be a harbinger of potential bottom-up impacts to the recruitment of other coastal small pelagic fish.
Data availability
The datasets analyzed during the current study (Fig. 2) are available on the ICES website (www.ices.dk) and in the following Git-hub repository (including the corresponding code): https://github.com/BjoernIlling/Moyano_et_al_2022_Fig.2.git.
Change history
18 January 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11160-022-09754-3
References
Akimova A, Hufnagl M, Kreus M, Peck MA (2016a) Modeling the effects of temperature on the survival and growth of North Sea cod (Gadus morhua) through the first year of life. Fish Oceanogr 25:193–209. https://doi.org/10.1111/fog.12145
Akimova A, Núñez-Riboni I, Kempf A, Taylor MH (2016b) Spatially-resolved influence of temperature and salinity on stock and recruitment variability of commercially important fishes in the North Sea. PLoS One 11:e0161917. https://doi.org/10.1371/journal.pone.0161917
Akimova A, Hufnagl M, Peck MA (2019) Spatiotemporal dynamics of predators and survival of marine fish early life stages: Atlantic cod (Gadus morhua) in the North Sea. Prog Oceanogr 176:102121. https://doi.org/10.1016/j.pocean.2019.102121
Alheit J, Pohlmann T, Casini M, Greve W, Hinrichs R, Mathis M, O’Driscoll K, Vorberg R, Wagner C (2012) Climate variability drives anchovies and sardines into the North and Baltic Seas. Prog Oceanogr 96:128–139
Allan BJM, Ray JL, Tiedemann M et al (2021) Quantitative molecular detection of larval Atlantic herring (Clupea harengus) in stomach contents of Atlantic mackerel (Scomber scombrus) marks regions of predation pressure. Sci Rep 11:5095. https://doi.org/10.1038/s41598-021-84545-7
Allan BJM, Browman HI, Shema S et al (2022) Increasing temperature and prey availability affect the growth and swimming kinematics of Atlantic herring (Clupea harengus) larvae. J Plankton Res 44:401–413. https://doi.org/10.1093/plankt/fbac014
Andersen JH, Carstensen J, Conley DJ et al (2017) Long-term temporal and spatial trends in eutrophication status of the Baltic Sea: Eutrophication in the Baltic Sea. Biol Rev 92:135–149. https://doi.org/10.1111/brv.12221
Aneer G (1987) High natural mortality of Baltic herring (Clupea harengus) eggs caused by algal exudates? Mar Biol 94:163–169
Aneer G, Nellbring S (1982) A SCUBA-diving investigation of Baltic herring (Clupea harengus membras L.) spawning grounds in the Askö-Landsort area, Northern Baltic proper. J Fish Biol 21:433–442
Arula T, Kotta J, Lankov A, Simm M, Põlme S (2012) Diet composition and feeding activity of larval spring-spawning herring: importance of environmental variability. J Sea Res 68:33–40. https://doi.org/10.1016/j.seares.2011.12.003
Arula T, Laur K, Simm M, Ojaveer H (2015) Dual impact of temperature on growth and mortality of marine fish larvae in a shallow estuarine habitat. Estuar Coast Shelf Sci 167:326–335. https://doi.org/10.1016/j.ecss.2015.10.004
Arula T, Raid T, Simm M, Ojaveer H (2016) Temperature-driven changes in early life-history stages influence the Gulf of Riga spring spawning herring (Clupea harengus m.) recruitment abundance. Hydrobiologia 767:125–135. https://doi.org/10.1007/s10750-015-2486-8
Bailey KM, Houde ED (1989) Predation on eggs and larvae of marine fishes and the recruitment problem. Adv Mar Biol 25:1–83
Balon EK (1975) Reproductive guilds of fishes: a proposal and definition. J Fish Res Board Can 32:821–864. https://doi.org/10.1139/f75-110
Baršienė J, Butrimavičienė L, Grygiel W, Lang T, Michailovas A, Jackūnas T (2014) Environmental genotoxicity and cytotoxicity in flounder (Platichthys flesus), herring (Clupea harengus) and Atlantic cod (Gadus morhua) from chemical munitions dumping zones in the southern Baltic Sea. Mar Environ Res 96:56–67
Bartsch J, Brander K, Heath M, Munk P, Richardson K, Svendsen E (1989) Modelling the advection of herring larvae in the North Sea. Nature 340(6235):632–636. https://doi.org/10.1038/340632a0
Bauer RK, Stepputtis D, Gräwe U, Zimmermann C, Hammer C (2013) Wind-induced variability in coastal larval retention areas: a case study on Western Baltic spring-spawning herring. Fish Oceanogr 22:388–399. https://doi.org/10.1111/fog.12029
Bauer RK, Gräwe U, Stepputtis D, Zimmermann C, Hammer C (2014) Identifying the location and importance of spawning sites of Western Baltic herring using a particle backtracking model. ICES J Mar Sci 71:499–509. https://doi.org/10.1093/icesjms/fst163
Bekkevold D, Helyar SJ, Limborg MT, Nielsen EE, Hemmer-Hansen J, Clausen LAW, Carvalho GR (2015) Gene-associated markers can assign origin in a weakly structured fish, Atlantic herring. ICES J Mar Sci 72:1790–1801. https://doi.org/10.1093/icesjms/fsu247
Berg F, Slotte A, Johannessen A, Kvamme C, Clausen LW, Nash RDM (2017) Comparative biology and population mixing among local, coastal and offshore Atlantic herring (Clupea harengus) in the North Sea, Skagerrak, Kattegat and Western Baltic. PLoS One 12:e0187374. https://doi.org/10.1371/journal.pone.0187374
Biester E (1979) Der Frühjahrshering Rügens, seine Rolle in der Fischerei der Ostsee und in den Übergangsgebieten zur Nordsee. PhD thesis, Wilhelm-Pieck Universität Rostock
Bils F, Moyano M, Aberle N, Hufnagl M, Alvarez-Fernandez S, Peck MA (2017) Exploring the microzooplankton–ichthyoplankton link: a combined field and modeling study of Atlantic herring (Clupea harengus) in the Irish Sea. J Plankton Res 39:147–163. https://doi.org/10.1093/plankt/fbw074
Bishop MA, Green SP (2001) Predation on Pacific herring (Clupea pallasi) spawn by birds in Prince William sound. Alaska 10:149–158. https://doi.org/10.1046/j.1054-6006.2001.00038.x
Bjørndal T (1989) Production in a schooling fishery: the case of the North Sea herring fishery. Land Econ 65(1):49–56
Blaxter JHS, Hempel G (1963) The influence of egg size on herring larvae (Clupea harengus L.). ICES J Mar Sci 28:211–240. https://doi.org/10.1093/icesjms/28.2.211
Bodenstein S (2012) Effects of ocean acidification and temperature on chloride cells in Atlantic herring (Clupea harengus) embryos and larvae. Diploma thesis, Christian-Albrechts-Universität zu Kiel. pp 82
Boyce DG, Petrie B, Frank KT (2021) Fishing, predation, and temperature drive herring decline in a large marine ecosystem. Ecol Evol. https://doi.org/10.1002/ece3.8411
Braum E (1973) Einflüsse chronischen exogenen Sauerstoffmangels auf die Embryogenese des Herings (Clupea harengus). Neth J Sea Res 7:363–375
Braum E (1985) Experiments on the respiration and in situ development of Baltic herring (Clupea harengus L.). Int Rev Gesamten Hydrobiol Berl 70:87–100
Brosset P, Smith AD, Plourde S et al (2020) A fine-scale multi-step approach to understand fish recruitment variability. Sci Rep 10:16064. https://doi.org/10.1038/s41598-020-73025-z
Bueno-Pardo J, Petitgas P, Kay S, Huret M (2020) Integration of bioenergetics in an individual-based model to hindcast anchovy dynamics in the Bay of Biscay. ICES J Mar Sci 77:655–667. https://doi.org/10.1093/icesjms/fsz239
Bull CD, Gregory SD, Rivot E et al (2022) The likely suspects framework: the need for a life cycle approach for managing Atlantic salmon (Salmo salar) stocks across multiple scales. ICES J Marine Sci. https://doi.org/10.1093/icesjms/fsac099
Busch A (1996) Transition from endogenous to exogenous nutrition:larval size parameters determining the start of external feeding and size of prey ingested by Ruegen spring herring Clupea harengus. Mar Ecol Prog Ser 130:39–46. https://doi.org/10.3354/meps130039
Cardinale M, Möllmann C, Bartolino V et al (2009) Effect of environmental variability and spawner characteristics on the recruitment of Baltic herring Clupea harengus populations. Mar Ecol Prog Ser 388:221–234. https://doi.org/10.3354/meps08125
Casini M, Bartolino V, Molinero J, Kornilovs G (2010) Linking fisheries, trophic interactions and climate: threshold dynamics drive herring Clupea harengus growth in the central Baltic Sea. Mar Ecol Prog Ser 413:241–252. https://doi.org/10.3354/meps08592
Catalán IA, Auch D, Kamermans P et al (2019) Critically examining the knowledge base required to mechanistically project climate impacts: a case study of Europe’s fish and shellfish. Fish Fish. https://doi.org/10.1111/faf.12359
Checkley DM (1982) Selective feeding by Atlantic herring (Clupea harengus) Larvae on Zooplankton in natural assemblages. Mar Ecol Prog Ser 9:245–253. https://doi.org/10.3354/MEPS009245
Checkley D, Roy C, Alheit J, Oozeki Y (eds) (2009) Climate change and small pelagic fish Edited by Claude Roy , Edited by Jürgen Alheit , Edited by Yoshioki Oozeki. Cambridge University Press, Cambridge, UK ; New York
Clausen LAW, Staehr K-J, Rindorf A, Mosegaard H (2015) Effect of spatial differences in growth on distribution of seasonally co-occurring herring Clupea harengus stocks. J Fish Biol 86:228–247. https://doi.org/10.1111/jfb.12571
Corten A (2013) Recruitment depressions in North Sea herring. ICES J Mar Sci 70:1–15. https://doi.org/10.1093/icesjms/fss187
Coumou D, Rahmstorf S (2012) A decade of weather extremes. Nat Clim Change 2:491–496. https://doi.org/10.1038/nclimate1452
Cury PM, Fromentin JM, Figuet S, Bonhommeau S (2014) Resolving Hjort’s Dilemma: How is recruitment related to spawning stock biomass in marine fish? Oceanography 27:4
Cushing DH (1975) Marine ecology and fisheries. Cambridge University Press, Cambridge
Daewel U, Peck MA, Schrum C (2011) Life history strategy and impacts of environmental variability on early life stages of two marine fishes in the North Sea: an individual-based modelling approach. Can J Fish Aquat Sci 68:426–443
Dahlke FT, Leo E, Mark FC, Pörtner H-O, Bickmeyer U, Frickenhaus S, Storch D (2017) Effects of ocean acidification increase embryonic sensitivity to thermal extremes in Atlantic cod, Gadus morhua. Glob Change Biol 23:1499–1510. https://doi.org/10.1111/gcb.13527
Diekmann ABS, Clemmesen C, St John MA, Paulsen M, Peck MA (2012) Environmental cues and constraints affecting the seasonality of dominant calanoid copepods in brackish, coastal waters: a case study of Acartia, Temora and Eurytemora species in the south-west Baltic. Mar Biol 159:2399–2414. https://doi.org/10.1007/s00227-012-1955-0
Dodson JJ, Daigle G, Hammer C, Polte P, Kotterba P, Winkler G, Zimmermann C (2019) Environmental determinants of larval herring (Clupea harengus) abundance and distribution in the western Baltic Sea: Larval herring ecology. Limnol Oceanogr. https://doi.org/10.1002/lno.11042
dos Santos Schmidt TC, Hay DE, Sundby S et al (2021) Adult body growth and reproductive investment vary markedly within and across Atlantic and Pacific herring: a meta-analysis and review of 26 stocks. Rev Fish Biol Fisheries 31:685–708. https://doi.org/10.1007/s11160-021-09665-9
Elliot M, Hemingway KL (2008) Fishes in estuaries. John Willey & Sons
Engelhard GH, Peck MA, Rindorf A et al (2014) Forage fish, their fisheries, and their predators: who drives whom? ICES J Mar Sci 71:90–104. https://doi.org/10.1093/icesjms/fst087
FAO (2018) The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals. FAO, Rome, Italy
Fiksen Ø, Folkvord A (1999) Modelling growth and ingestion processes in herring Clupea harengus larvae. Mar Ecol Prog Ser 184:273–289
Finke A, von Nordheim L, Kotterba P, Polte P (2022) Impact of spawn concentrations on Atlantic herring (Clupea harengus) egg survival in Baltic Sea inshore spawning areas. Estuar Coast Shelf Sci. https://doi.org/10.1016/j.ecss.2022.107961
Franke A, Clemmesen C (2011) Effect of ocean acidification on early life stages of Atlantic herring (Clupea harengus L.). Biogeosciences 8:3697–3707. https://doi.org/10.5194/bg-8-3697-2011
Frommel AY, Maneja R, Lowe D, Pascoe CK, Geffen AJ, Folkvord A, Piatkowski U, Clemmesen C (2014) Organ damage in Atlantic herring larvae as a result of ocean acidification. Ecol Appl 24:1131–1143. https://doi.org/10.1890/13-0297.1
Fuiman LA, Magurran AE (1994) Development of predator defences in fishes. Rev Fish Biol Fish 4:145–183. https://doi.org/10.1007/BF00044127
Garcia T, Planque B, Arneberg P, Bogstad B, Skagseth Ø, Tiedemann M (2020) An appraisal of the drivers of Norwegian spring-spawning herring (Clupea harengus) recruitment. Fish Oceanogr. https://doi.org/10.1111/fog.12510
Geffen AJ (2009) Advances in herring biology: from simple to complex, coping with plasticity and adaptability. ICES J Mar Sci 66:1688–1695. https://doi.org/10.1093/icesjms/fsp028
Griffin FJ, Smith EH, Vines CA, Cherr GN (2009) Impacts of suspended sediments on fertilization, embryonic development and early larval life stages of the Pacific herring, Clupea pallasi. Biol Bull 216:176–187
Gröger JP, Hinrichsen H-H, Polte P (2014) Broad-scale climate influences on spring-spawning herring (Clupea harengus L.) recruitment in the Western Baltic Sea. PLoS One. https://doi.org/10.1371/journal.pone.0087525
Gröhsler T, Oeberst R, Schaber M, Larson N, Kornilovs G (2013) Discrimination of western Baltic spring-spawning and central Baltic herring (Clupea harengus L.) based on growth vs. natural tag information. ICES J Mar Sci 70:1108–1117. https://doi.org/10.1093/icesjms/fst064
Gröhsler T, Müller H (2004) Updated Information on Maturity Ogives for Western Baltic Herring. In: Herring Assessment Working Group for the Area South of 62° N (HAWG), 09.-18.03.2004, Copenhagen, pp 12
Hansen P-D, von Westernhagen H, Rosenthal H (1985) Chlorinated hydrocarbons and hatching success in Baltic herring spring spawners. Mar Environ Res 15:59–76
Hare JA (2014) The future of fisheries oceanography lies in the pursuit of multiple hypotheses. ICES J Mar Sci 71:2343–2356
HELCOM 2018. HELCOM Assessment on maritime activities in the Baltic Sea 2018. Baltic Sea Environment Proceedings No.152. Helsinki Commission, Helsinki. 253pp. This report is available at www.helcom.fi (publications).
Hempel G, Schubert K (1968) Sterblichkeitsbestimmungen an einem Eiklumpen des Nordsee-Herings (Clupea harengus L.). Ber Dt Wiss Komm Meeresforsch 20:79–83
Hesse J. (2010) Quantitative and qualitative analyses of the food consumption of Clupea harengus L. larvae in the Greifswalder Bodden and Strelasund, Baltic Sea, Germany. MSc Thesis, University of Rostock
Hinkelmann A (1897) Über die im Jahre 1896 ausgeführte Versuchsfischerei im Kaiser Wilhelm-Kanal. Mitteilungen Des Deutschen Seefischereivereins 13:242–245
Hinrichsen H-H, Kühn W, Peck MA, Voss R (2012) The impact of physical and biological factors on the drift and spatial distribution of larval sprat: a comparison of the Baltic and North Seas. Prog Oceanogr 107:47–60. https://doi.org/10.1016/j.pocean.2012.05.004
Hixon MA, Johnson DW, Sogard SM (2014) BOFFFFs: on the importance of conserving old-growth age structure in fishery populations. ICES J Mar Sci 71:2171–2185. https://doi.org/10.1093/icesjms/fst200
Hjort J (1914) Fluctuations in the great fisheries of northern Europe viewed in the light of biological research. Rapp P-V Reun Cons Int Explor Mer 20:1–228
Hobday AJ, Spillman CM, Eveson JP, Hartog JR, Zhang X, Brodie S (2018) A framework for combining seasonal forecasts and climate projections to aid risk management for fisheries and aquaculture. Front Mar Sci 5:137. https://doi.org/10.3389/fmars.2018.00137
Houde ED (2008) Emerging from Hjort´s shadow. J Northwest Atl Fish Sci 41:53–70
Huang AT, Alter K, Polte P, Peck MA (2022) Disentangling seasonal from maternal effects on egg characteristics in western Baltic spring-spawning herring Clupea harengus. J Fish Biol. https://doi.org/10.1111/jfb.15210
Hufnagl M, Peck MA (2011) Physiological individual-based modelling of larval Atlantic herring (Clupea harengus) foraging and growth: insights on climate-driven life-history scheduling. ICES J Mar Sci 68:1170–1188. https://doi.org/10.1093/icesjms/fsr078
Hufnagl M, Peck MA, Nash RDM, Dickey-Collas M (2015) Unravelling the Gordian knot! Key processes impacting overwintering larval survival and growth: a North Sea herring case study. Prog Oceanogr 138:486–503. https://doi.org/10.1016/j.pocean.2014.04.029
Huse G, Fiksen Ø (2010) Modelling encounter rates and distribution of mobile predators and prey. Prog Oceanogr 84:93–104. https://doi.org/10.1016/j.pocean.2009.09.011
ICES (2018a) Report of the Benchmark Workshop on Pelagic Stocks (WKPELA 2018a), 12–16 February 2018a. ICES HQ, Copenhagen, Denmark. ICES CM 2018a/ACOM:32. pp 313
ICES (2018b) Report of the workshop on mixing of western and central Baltic herring stocks (WKMixHER). 11–13 September 2018b, Gdynia, Poland
ICES. 2019. Working Group on Multispecies Assessment Methods (WGSAM). ICES Scientific Reports. 1:91. 320 pp. https://doi.org/10.17895/ices.pub.5758
ICES (2021a) Baltic Fisheries Assessment Working Group (WGBFAS). ICES Scientific Reports. 3:53. 717 pp. Annex 5: Herring (Clupea harengus) in Subdivision 28.1 (Gulf of Riga) https://doi.org/10.17895/ices.pub.8187
ICES (2021b) Herring Assessment Working Group for the Area South of 62° N (HAWG) ICES Scientific Reports. 3:12. 779 pp. https://doi.org/10.17895/ices.pub.8214
Ihde TF, Townsend HM (2017) Accounting for multiple stressors influencing living marine resources in a complex estuarine ecosystem using an Atlantis model. Ecol Model 365:1–9. https://doi.org/10.1016/j.ecolmodel.2017.09.010
Iles TD, Sinclair M (1982) Atlantic herring: Stock discreteness and abundance. Science 215:627–633. https://doi.org/10.1126/science.215.4533.627
Illing B, Moyano M, Niemax J, Peck MA (2015) Direct effects of microalgae and protists on herring (Clupea harengus) yolk sac larvae. PLoS One 10:e0129344. https://doi.org/10.1371/journal.pone.0129344
Illing B, Moyano M, Hufnagl M, Peck MA (2016) Projected habitat loss for Atlantic herring in the Baltic Sea. Mar Environ Res 113:164–173. https://doi.org/10.1016/j.marenvres.2015.12.007
Illing B, Moyano M, Berg J, Hufnagl M, Peck MA (2018) Behavioral and physiological responses to prey match-mismatch in larval herring. Estuar Coast Shelf Sci 201:82–94. https://doi.org/10.1016/j.ecss.2016.01.003
Incardona JP, Vines CA, Anulacion BF et al (2012) Unexpectedly high mortality in Pacific herring embryos exposed to the 2007 Cosco Busan oil spill in San Francisco Bay. Proc Natl Acad Sci 109:E51–E58. https://doi.org/10.1073/pnas.1108884109
IPCC (2019) Summary for policymakers. In: Pörtner HO, Roberts DC, Masson-Delmotte V, Zhai P, Tignor M, Poloczanska E, Mintenbeck K, Alegría A, Nicolai M, Okem A, Petzold J, Rama B, Weyer NM (eds) IPCC Special Report on the Ocean and Cryosphere in a Changing Climate
Janke I (2019) Origin of winter herring (Clupea harengus, L.) larvae in coastal Baltic Sea spawning grounds. MSc Thesis, University of Rostock, Faculty of Mathematics and Natural Sciences
Kändler R, Dutt S (1958) Fecundity of Baltic herring. Rapp p-v Réun Cons Int Explor Mer 143:99–108
Kanstinger P, Beher J, Grenzdörffer G, Hammer C, Huebert KB, Stepputis D, Peck MA (2018) What is left? Macrophyte meadows and Atlantic herring (Clupea harengus) spawning sites in the Greifswalder Bodden, Baltic Sea. Estuar Coast Shelf Sci 201:72–81. https://doi.org/10.1016/j.ecss.2016.03.004
Karl H, Ruoff U (2007) Dioxins, dioxin-like PCBs and chloroorganic contaminants in herring, Clupea harengus, from different fishing grounds of the Baltic Sea. Chemosphere 67:590–595
Karp MA, Peterson JO, Lynch PD et al (2019) Accounting for shifting distributions and changing productivity in the development of scientific advice for fishery management. ICES J Marine Sci. https://doi.org/10.1093/icesjms/fsz048
Kinne O, Rosenthal H (1967) Effects of sulfuric water pollutants on fertilization, embryonic development and larvae of the herring, Clupea harengus. Mar Biol 1:65–83
Kiørboe T, Munk P (1987) Feeding and growth of larval herring, Clupea harengus, in relation to density of copepod nauplii. Environ Biol Fishes 17:133–139. https://doi.org/10.1007/BF00001743
Kiørboe T, Frantsen E, Jensen C, Sørensen G (1981) Effects of suspended sediment on development and hatching of herring (Clupea harengus) eggs. Estuar Coast Shelf Sci 13:107–111
Kiørboe T, Munk P, Støttrup JG (1985) First feeding by larval herring Clupea harengus L. Dana 95–107
Klinkhardt M (1986) Ergebnisse von Untersuchungen zur Schlupf- und Dottersackphase der Larven von Rügenschen Frühjahrsheringen (Clupea harengus L.). Fischereiforschung 24:28–30
Koenigstein S, Mark FC, Gößling-Reisemann S, Reuter H, Poertner H-O (2016) Modelling climate change impacts on marine fish populations: process-based integration of ocean warming, acidification and other environmental drivers. Fish Fish 17:972–1004. https://doi.org/10.1111/faf.12155
Koenigstein S, Dahlke FT, Stiasny MH, Storch D, Clemmesen C, Pörtner H-O (2018) Forecasting future recruitment success for Atlantic cod in the warming and acidifying Barents Sea. Glob Change Biol 24:526–535. https://doi.org/10.1111/gcb.13848
Kotterba P, Kühn C, Hammer C, Polte P (2014) Predation of threespine stickleback (Gasterosteus aculeatus) on the eggs of Atlantic herring (Clupea harengus) in a Baltic Sea lagoon. Limnol Oceanogr 59:578–587. https://doi.org/10.4319/lo.2014.59.2.0578
Kotterba P, Moll D, Hammer C, Peck MA, Oesterwind D, Polte P (2017a) Predation on Atlantic herring (Clupea harengus) eggs by the resident predator community in coastal transitional waters: predation on Atlantic herring eggs. Limnol Oceanogr. https://doi.org/10.1002/lno.10594
Kotterba P, Moll D, von Nordheim L, Peck MA, Oesterwind D, Polte P (2017b) Predation on larval Atlantic herring (Clupea harengus) in inshore waters of the Baltic Sea. Estuar Coast Shelf Sci 198:1–11. https://doi.org/10.1016/j.ecss.2017.08.017
Kotterba P (2015) Atlantic herring (Clupea harengus) within the Estuarine Foodweb of a Southern Baltic Sea lagoon. University of Hamburg
Kuparinen A, Keith DM, Hutchings JA (2014) Increased environmentally driven recruitment variability decreases resilience to fishing and increases uncertainty of recovery. ICES J Mar Sci 71:1507–1514. https://doi.org/10.1093/icesjms/fsu021
Laine P, Rajasilta M (1999) The hatching success of Baltic herring eggs and its relation to female condition. J Exp Mar Biol Ecol 237:61–73. https://doi.org/10.1016/S0022-0981(98)00213-5
Lehmann A, Krauss W, Hinrichsen H-H (2002) Effects of remote and local atmospheric forcing on circulation and upwelling in the Baltic Sea. Tellus Dyn Meteorol Oceanogr 54:299–316. https://doi.org/10.3402/tellusa.v54i3.12138
Leipe T (1985) Zur Nahrungsökologie der Eisente (Clanguela hyemalis) im Greifswalder Bodden. Beitra¨ge Zur Vogelkd 31:121–140.
Leo E, Dahlke FT, Storch D, Pörtner H-O, Mark FC (2018) Impact of Ocean Acidification and Warming on the bioenergetics of developing eggs of Atlantic herring Clupea harengus. Conserv Physiol. https://doi.org/10.1093/conphys/coy050
Lindegren M, Östman Ö, Gårdmark A (2011) Interacting trophic forcing and the population dynamics of herring. Ecology 92:1407–1413. https://doi.org/10.1890/10-2229.1
Lindén O (1978) Biological effect of oil on early development of the Baltic herring Clupea harengus membras. Mar Biol 45:273–283
Lok EK, Kirk M, Esler D, Boyd WS (2008) Movements of pre-migratory surf and white-winged scoters in response to Pacific herring spawn. Waterbirds 31:385–393. https://doi.org/10.1675/1524-4695-31.3.385
Maneja RH, Frommel AY, Browman HI, Geffen AJ, Folkvord A, Piatkowski U, Durif CMF, Bjelland R, Skiftesvik AB, Clemmesen C (2015) The swimming kinematics and foraging behavior of larval Atlantic herring (Clupea harengus L.) are unaffected by elevated pCO2. J Exp Mar Biol Ecol 466:42–48. https://doi.org/10.1016/j.jembe.2015.02.008
Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska MA, Bange HW, Hansen HP, Körtzinger A (2013) Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar Biol 160:1875–1888. https://doi.org/10.1007/s00227-012-1954-1
Messieh SN, Rosenthal H (1989) Mass mortality of herring eggs on spawning beds on and near Fisherman’s Bank, Gulf of St. Lawrence. Canada Aquat Living Resour 2:1–8
Moll D, Kotterba P, von Nordheim L, Polte P (2018) Storm-induced atlantic herring (Clupea harengus) egg mortality in Baltic Sea inshore spawning areas. Estuaries Coasts 41:1–12. https://doi.org/10.1007/s12237-017-0259-5
Moll D, Kotterba P, Jochum KP, von Nordheim L, Polte P (2019) Elemental inventory in fish otoliths reflects natal origin of Atlantic Herring (Clupea harengus) from Baltic Sea juvenile areas. Front Mar Sci 6:191. https://doi.org/10.3389/fmars.2019.00191
Moll D (2018) Contribution of coastal nursery areas to the spring-spawning population of Atlantic herring (Clupea harengus) in the Western Baltic Sea. PhD thesis, University of Hamburg
Möller H (1984) Reduction of a larval herring population by jellyfish predator. Science 224:621–622. https://doi.org/10.1126/science.224.4649.621
Möllmann C, Muller-Karulis B, Kornilovs G, St John MA (2008) Effects of climate and overfishing on zooplankton dynamics and ecosystem structure: regime shifts, trophic cascade, and feedback loops in a simple ecosystem. ICES J Mar Sci 65:302–310. https://doi.org/10.1093/icesjms/fsm197
Montagnes DJS, Dower JF, Figueiredo GM (2010) The protozooplankton-ichthyoplankton trophic link: an overlooked aspect of aquatic food webs. J Eukaryot Microbiol 57:223–228. https://doi.org/10.1111/j.1550-7408.2010.00476.x
Moyano M, Illing B, Peschutter P, Huebert KB, Peck MA (2016) Thermal impacts on the growth, development and ontogeny of critical swimming speed in Atlantic herring larvae. Comp Biochem Physiol A Mol Integr Physiol 197:23–34. https://doi.org/10.1016/j.cbpa.2016.02.020
Moyano M, Candebat C, Ruhbaum Y et al (2017) Effects of warming rate, acclimation temperature and ontogeny on the critical thermal maximum of temperate marine fish larvae. PLoS ONE 12:e0179928. https://doi.org/10.1371/journal.pone.0179928
Moyano M, Illing B, Christiansen L, Peck MA (2018) Linking rates of metabolism and growth in marine fish larvae. Mar Biol. https://doi.org/10.1007/s00227-017-3252-4
Moyano M, Illing B, Polte P, Kotterba P, Zablotski Y, Gröhsler T, Hüdepohl P, Cooke SJ, Peck MA (2020) Linking individual physiological indicators to the productivity of fish populations: a case study of Atlantic herring. Ecol Indic 113:106146. https://doi.org/10.1016/j.ecolind.2020.106146
Muhling BA, Brodie S, Smith JA et al (2020) Predictability of species distributions deteriorates under novel environmental conditions in the California current system. Front Mar Sci 7:589. https://doi.org/10.3389/fmars.2020.00589
Munk P, Kiørboe T (1985) Feeding behaviour and swimming activity of larval herring (Clupea harengus) in relation to density of copepod nauplii. Mar Ecol Progres Ser 24:15–21
Munkes B (2005) Eutrophication, phase shift, the delay and the potential return in the Greifswalder Bodden, Baltic Sea. Aquat Sci 67:372–381. https://doi.org/10.1007/s00027-005-0761-x
Nash RDM, Dickey-Collas M (2005) The influence of life history dynamics and environment on the determination of year class strength in North Sea herring (Clupea harengus L.). Fish Oceanogr 14:279–291. https://doi.org/10.1111/j.1365-2419.2005.00336.x
Neb KE (1952) Untersuchungen zur Fortpflanzung und Wachstum an den Heringen der westlichen Ostsee mit besonderer Berücksichtigung der Kieler
Nielsen JR, Lundgren B, Jensen TF, Stæhr K-J (2001) Distribution, density and abundance of the western Baltic herring (Clupea harengus) in the sound (ICES Subdivision 23) in relation to hydrographical features. Fish Res 50:235–258. https://doi.org/10.1016/S0165-7836(00)00220-4
Oeberst R, Klenz B, Gröhsler T, Dickey-Collas M, Nash RDM, Zimmermann C (2009) When is year-class strength determined in western Baltic herring? ICES J Mar Sci 66:1667–1672. https://doi.org/10.1093/icesjms/fsp143
Paradis AR, Pepin P, Brown JA (1996) Vulnerability of fish eggs and larvae to predation: Review of the influence of the relative size of prey and predator. Can J Fish Aquat Sci 53:1226–1235. https://doi.org/10.1139/f96-051
Parmanne R, Rechlin O, Sjöstrand B (1994) Status and future of herring and sprat stocks in the Baltic Sea. Dana 10:29–59
Parrish BB, Saville A, Craig RE, Blaxter JHS, Priestley R (1959) Observations on herring spawning and larval distribution in the Firth of Clyde in 1958. J Mar Biol Assoc U K 38:445–453
Paulsen M, Clemmesen C, Malzahn AM (2014a) Essential fatty acid (docosahexaenoic acid, DHA) availability affects growth of larval herring in the field. Mar Biol 161:239–244. https://doi.org/10.1007/s00227-013-2313-6
Paulsen M, Hammer C, Malzahn AM, Polte P, von Dorrien C, Clemmesen C (2014b) Nutritional situation for larval Atlantic herring (Clupea harengus L.) in two nursery areas in the western Baltic Sea. ICES J Mar Sci 71:991–1000. https://doi.org/10.1093/icesjms/fst168
Paulsen M, Clemmesen C, Hammer C, Polte P, Malzahn AM (2016) Food-limited growth of larval Atlantic herring Clupea harengus recurrently observed in a coastal nursery area. Helgol Mar Res. https://doi.org/10.1186/s10152-016-0470-y
Peck MA, Hufnagl M (2012) Can IBMs tell us why most larvae die in the sea? Model sensitivities and scenarios reveal research needs. J Mar Syst 93:77–93. https://doi.org/10.1016/j.jmarsys.2011.08.005
Peck MA, Huebert KB, Llopiz JK (2012a) Intrinsic and extrinsic factors driving match-mismatch dynamics during the early life history of marine fishes. Adv Ecol Res 47:177–302
Peck MA, Kanstinger P, Holste L, Martin M (2012b) Thermal windows supporting survival of the earliest life stages of Baltic herring (Clupea harengus). ICES J Mar Sci 69:529–536
Peck MA, Reglero P, Takahashi M, Catalán IA (2013) Life cycle ecophysiology of small pelagic fish and climate-driven changes in populations. Prog Oceanogr 116:220–245. https://doi.org/10.1016/j.pocean.2013.05.012
Peck MA, Alheit J, Bertrand A, Catalán IA, Garrido S, Moyano M, Rykaczewski RR, Takasuka A, van der Lingen CD (2021) Small pelagic fish in the new millennium: a bottom-up view of global research effort. Prog Oceanogr 191:102494. https://doi.org/10.1016/j.pocean.2020.102494
Pécuchet L, Nielsen JR, Christensen A (2015) Impacts of the local environment on recruitment: a comparative study of North Sea and Baltic Sea fish stocks. ICES J Mar Sci 72:1323–1335. https://doi.org/10.1093/icesjms/fsu220
Pepin P, Penney RW (1997) Patterns of prey size and taxonomic composition in larval fish: are there general size-dependent models? J Fish Biol 51:84–100. https://doi.org/10.1111/j.1095-8649.1997.tb06094.x
Pereira R, Teodósio MA, Garrido S (2014) An experimental study of Aurelia aurita feeding behaviour: inference of the potential predation impact on a temperate estuarine nursery area. Estuar Coast Shelf Sci 146:102–110. https://doi.org/10.1016/j.ecss.2014.05.026
Perttilä M, Tervo V, Parmanne R (1982) Age dependence of the concentrations of harmful substances in Baltic herring (Clupea harengus). Chemosphere 11:1019–1026
Peschutter, JGR (2008) Ernährungszustand von Fischlarven in der Kieler Förde und im Nord-Ostsee-Kanal. Diplomarbeit Universität Kiel, pp 134
Polte P, Asmus H (2006) Intertidal seagrass beds (Zostera noltii) as spawning grounds for transient fishes in the Wadden Sea. Mar Ecol Prog Ser 312:235–243. https://doi.org/10.3354/meps312235
Polte P, Kotterba P, Hammer C, Gröhsler T (2014) Survival bottlenecks in the early ontogenesis of Atlantic herring (Clupea harengus L.) in coastal lagoon spawning areas of the western Baltic Sea. ICES J Mar Sci 71:982–990. https://doi.org/10.1093/icesjms/fst050
Polte P, Kotterba P, Moll D, von Nordheim L (2017) Ontogenetic loops in habitat use highlight the importance of littoral habitats for early life-stages of oceanic fishes in temperate waters. Sci Rep 7:42709. https://doi.org/10.1038/srep42709
Polte P, Gröhsler T, Kotterba P et al (2021) Reduced reproductive success of Western baltic herring (Clupea harengus) as a response to warming winters. Front Mar Sci 8:10. https://doi.org/10.3389/fmars.2021.589242
Rahikainen M, Hoviniemi KM, Mäntyniemi S, Vanhatalo J, Helle I, Lehtiniemi M, Pönni J, Kuikka S (2017) Impacts of eutrophication and oil spills on the Gulf of Finland herring stock. Can J Fish Aquat Sci 74:1218–1232
Rajasilta M (1993) Spawning of herring (Clupea harengus membras L.) in the Archipelago sea. ICES J Mar Sci 50:233–246. https://doi.org/10.1006/jmsc.1993.1026
Rajasilta M, Eklund J, Kääriä J, Ranta-Aho K (1989) The deposition and mortality of the eggs of the Baltic herring Clupea harengus membras L., on different substrates in the south-west archipelago of Finland. J Fish Biol 34:417–427
Rajasilta M, Laine P, Eklund J (2006) Mortality of herring eggs on different algal substrates (Furcellaria spp. and Cladophora spp.) in the Baltic Sea – an experimental study. Hydrobiologia 554:127–130
Rajasilta M, Hänninen J, Laaksonen L, Laine P, Suomela J-P, Vuorinen I, Mäkinen K (2019) Influence of environmental conditions, population density, and prey type on the lipid content in Baltic herring (Clupea harengus membras) from the northern Baltic Sea. Can J Fish Aquat Sci 76:576–585. https://doi.org/10.1139/cjfas-2017-0504
Ramirez-Romero E, Molinero JC, Paulsen M, Javidpour J, Clemmesen C, Sommer U (2018) Quantifying top-down control and ecological traits of the scyphozoan Aurelia aurita through a dynamic plankton model. J Plankton Res. https://doi.org/10.1093/plankt/fby041
Reindl AR, Falkowska L, Grajewska A (2015) Chlorinated herbicides in fish, birds and mammals in the Baltic Sea. Water Air Soil Pollut 226:276. https://doi.org/10.1007/s11270-015-2536-x
Reusch TBH, Dierking J, Andersson HC et al (2018) The Baltic Sea as a time machine for the future coastal ocean. Sci Adv. https://doi.org/10.1126/sciadv.aar8195
Richardson DE, Hare JA, Fogarty MJ, Link JS (2011) Role of egg predation by haddock in the decline of an Atlantic herring population. Proc Natl Acad Sci USA 108:13606–13611. https://doi.org/10.1073/pnas.1015400108
Robert D, Takasuka A, Nakatsuka S, Kubota H, Oozeki Y, Nishida H, Fortier L (2010) Predation dynamics of mackerel on larval and juvenile anchovy: is capture success linked to prey condition? Fish Sci 76:183–188. https://doi.org/10.1007/s12562-009-0205-y
Robert D, Murphy HM, Jenkins GP, Fortier L (2014) Poor taxonomical knowledge of larval fish prey preference is impeding our ability to assess the existence of a “critical period” driving year-class strength. ICES J Mar Sci 71:2042–2052. https://doi.org/10.1093/icesjms/fst198
Rosenthal H (1971) Wirkungen von “Rotschlamm” auf Embryonen und Larven des Herings Clupea harengus. Helgol Mar Res 22:366–376
Rosenthal H, Alderdice DF (1976) Sublethal effects of environmental stressor, natural and pollutional, on marine fish eggs and larvae. J Fish Res Board Can 33:2047–2065
Rosenthal H, Kamp M, Waller U (1995) Abschlußbericht zur Begleituntersuchung Terminal 3. Christian-Albrechts University Kiel, Germany, 1–49
Sætre R (2002) Factors affecting the recruitment variability of the Norwegian spring-spawning herring (Clupea harengus L.). ICES J Mar Sci 59:725–736. https://doi.org/10.1006/jmsc.2002.1180
Scabell J (1988) Der Rügensche Frühjahrshering - das Laichgeschen. PhD thesis, Rostock University
Schaber M, Grohsler T (2021) Survey Report for FRV SOLEA/German Acoustic Autumn Survey (GERAS) 1 – 21 October 2020. In: ICES.2021. Working group of international pelagic surveys (WGIPS). ICES scientific reports. 340, pp 481. https://doi.org/10.17895/ices.pub.8055
Schneider G, Behrends G (1998) Top-down control in a neritic plankton system by Aurelia aurita medusae — a summary. Ophelia 48:71–82. https://doi.org/10.1080/00785236.1998.10428677
Schubert S, Keddig N, Gerwinski W, Neukirchen J, Kammann U, Haarich M, Hanel R, Theobald N (2016) Persistent organic pollutants in Baltic herring (Clupea harengus)—an aspect of gender. Environ Monit Assess 188:368
Shelton AO, Mangel M (2011) Fluctuations of fish populations and the magnifying effects of fishing. Proc Natl Acad Sci 108:7075–7080. https://doi.org/10.1073/pnas.1100334108
Sinclair M (2009) Herring and ICES: a historical sketch of a few ideas and their linkages. ICES J Mar Sci 66:1652–1661. https://doi.org/10.1093/icesjms/fsp115
Siple MC, Koehn LE, Johnson KF et al (2021) Considerations for management strategy evaluation for small pelagic fishes. Fish Fish 22:1167–1186. https://doi.org/10.1111/faf.12579
Skaret G, Bachiller E, Langøy H, Stenevik EK (2015) Mackerel predation on herring larvae during summer feeding in the Norwegian Sea. ICES J Mar Sci J Cons 72:2313–2321. https://doi.org/10.1093/icesjms/fsv087
Skern-Mauritzen M, Ottersen G, Handegard NO, Huse G, Dingsør GE, Stenseth NC, Kjesbu OS (2016) Ecosystem processes are rarely included in tactical fisheries management. Fish Fish 17:165–175. https://doi.org/10.1111/faf.12111
Spittler P, Brenning U, Arlt G (1990) Protozoans—the first food of larval herring (Clupea harengus L.)? Int Rev Ges Hydrobiol Hydrogr 75:597–603. https://doi.org/10.1002/IROH.19900750503
Sswat M, Stiasny MH, Taucher J, Algueró-Muñiz M, Bach LT, Jutfelt F, Riebesell U, Clemmesen C (2018a) Food web changes under ocean acidification promote herring larvae survival. Nat Ecol Evol 2:836–840. https://doi.org/10.1038/s41559-018-0514-6
Sswat M, Stiasny MH, Jutfelt F, Riebesell U, Clemmesen C (2018b) Growth performance and survival of larval Atlantic herring, under the combined effects of elevated temperatures and CO2. PLoS One 13:e0191947. https://doi.org/10.1371/journal.pone.0191947
Stempniewicz L (1995) Feeding ecology of the Long-tailed Duck Clangula hyemalis wintering in the Gulf of Gdansk (southern Baltic Sea)
Subbey S, Devine JA, Schaarschmidt U, Nash RDM (2014) Modelling and forecasting stock–recruitment: current and future perspectives. ICES J Mar Sci 71:2307–2322. https://doi.org/10.1093/icesjms/fsu148
Szuwalski CS, Vert-Pre KA, Punt AE, Branch TA, Hilborn R (2015) Examining common assumptions about recruitment: a meta-analysis of recruitment dynamics for worldwide marine fisheries. Fish Fish 16:633–648. https://doi.org/10.1111/faf.12083
Takasuka A, Aoki I, Oozeki Y (2007) Predator-specific growth-selective predation on larval Japanese anchovy Engraulis japonicus. Mar Ecol Prog Ser 350:99–107. https://doi.org/10.3354/meps07158
Thomsen J, Gutowska MA, Saphörster J et al (2010) Calcifying invertebrates succeed in a naturally CO2-rich coastal habitat but are threatened by high levels of future acidification. Biogeosciences 7:3879–3891. https://doi.org/10.5194/bg-7-3879-2010
Tibbo SN, Scarratt DJ, McMullon PWG (1963) An investigation of herring (Clupea harengus L.) spawning using free-diving techniques. J Fish Res Board Can 20:1067–1079. https://doi.org/10.1139/f63-071
Tilves U, Fuentes V, Milisenda G, Parrish C, Vizzini S, Sabatés A (2018) Trophic interactions of the jellyfish Pelagia noctiluca in the NW Mediterranean: evidence from stable isotope signatures and fatty acid composition. Mar Ecol Prog Ser 591:101–116. https://doi.org/10.3354/meps12332
Titelman J, Hansson LJ (2006) Feeding rates of the jellyfish Aurelia aurita on fish larvae. Mar Biol 149:297–306. https://doi.org/10.1007/s00227-005-0200-5
Veloza AJ, Chu F-LE, Tang KW (2006) Trophic modification of essential fatty acids by heterotrophic protists and its effects on the fatty acid composition of the copepod Acartia tonsa. Mar Biol 148:779–788. https://doi.org/10.1007/s00227-005-0123-1
Villalobos C, Love BA, Olson MB (2020) Ocean acidification and ocean warming effects on pacific herring (Clupea pallasi) early life stages. Front Mar Sci 7:597899. https://doi.org/10.3389/fmars.2020.597899
Von Nordheim L (2019) Effects of coastal habitat characteristics on the reproduction of Baltic herring (Clupea harengus). Hamburg University, Hamburg
Von Dorrien C, Hammer C, Zimmermann C, Stepputtis D, Stuermer IW, Kotterba P, Polte P (2013) A review on herring, Clupea harengus (Actinopterygii: Clupeiformes: Clupeidae) recruitment and early life stage ecology in the Western Baltic Sea. Acta Ichthyol Piscat 43:169–182. https://doi.org/10.3750/AIP2013.43.3.01
von Nordheim L, Kotterba P, Moll D, Polte P (2018) Impact of spawning substrate complexity on egg survival of Atlantic herring (Clupea harengus, L.) in the Baltic Sea. Estuaries Coasts 41:549–559. https://doi.org/10.1007/s12237-017-0283-5
von Nordheim L, Kotterba P, Moll D, Polte P (2020) Lethal effect of filamentous algal blooms on Atlantic herring (Clupea harengus) eggs in the Baltic Sea. Aquat Conserv Mar Freshw Ecosyst 30:1362–1372. https://doi.org/10.1002/aqc.3329
von Westernhagen H, Dethlefsen V, Rosenthal H (1979) Combined effects of cadmium, copper and lead on developing herring eggs and larvae. Helgol Mar Res 32:257–278
Voss R, Peck MA, Hinrichsen H-H, Clemmesen C, Baumann H, Stepputtis D, Bernreuther M, Schmidt JO, Temming A, Köster FW (2012) Recruitment processes in Baltic sprat – a re-evaluation of GLOBEC Germany hypotheses. Prog Oceanogr 107:61–79. https://doi.org/10.1016/j.pocean.2012.05.003
Weber W (1971) Die Laichplatze des herings (Clupea harengus L.) der westlichen Ostsee. Kiel Meeresforsch 27:194–208
Werner FE, Quinlan JA, Lough RG, Lynch DR (2001) Spatially-explicit individual based modeling of marine populations: a review of the advances in the 1990s. Sarsia 86:405–410
von Westernhagen H (1988) Sublethal effects of pollutants on fish eggs and larvae. In: Hoar WS, Randall DJ (eds.) Fish Physiology Series, pp 253–346
Wiegleb J, Kotterba P, Hammer C, Oesterwind D (2018) Predation of the round goby (Neogobius melanostomus Pallas, 1814) on Atlantic herring eggs in the Western Baltic Sea. Mar Biol Res 14:989–1003. https://doi.org/10.1080/17451000.2019.1577977
Zander CD, Reimer LW (2002) Parasitism at the ecosystem level in the Baltic Sea. Parasitology 124(7):119–135. https://doi.org/10.1017/S0031182002001567
Zimmermann F, Claireaux M, Enberg K (2019) Common trends in recruitment dynamics of north-east Atlantic fish stocks and their links to environment, ecology and management. Fish Fish 20:518–536. https://doi.org/10.1111/faf.12360
Zwolinski JP, Emmett RL, Demer DA (2011) Predicting habitat to optimize sampling of Pacific sardine (Sardinops sagax). ICES J Mar Sci 68:867–879. https://doi.org/10.1093/icesjms/fsr038
Žydelis R, Esler D (2005) Response of wintering Steller’s eiders to herring spawn. Waterbirds 28:344–350. https://doi.org/10.1675/1524-4695(2005)028[0344:ROWSET]2.0.CO;2
Acknowledgements
The authors would like to acknowledge all the students and the staff supporting the field work and laboratory experiments related to herring research over the past 20 years at the Thünen Institute, GEOMAR, and University of Hamburg. We also thank Thomas Eikeland Fiskå and Nakula Plantener for their help with the graphical abstract and Figure 1, respectively, as well as two anonymous reviewers who helped improve the manuscript. The authors are grateful for comments received from members of the ICES-PICES Working Group on Small Pelagic Fish (ICES WGSPF/PICES WG23)
Funding
Open access funding provided by University of Agder. This work has been partially funded by the German Research Foundation (THRESHOLDS, MO 2873/3–1, and postdoctoral fellowship to B.I. 220/3–1), the joint Baltic Sea Research and Development Programme (BONUS BIO-C3, grant no. 03F0682B; BONUS-INSPIRE, grant no. 03F0681B) and the German Federal Ministry of Education and Research (DAM-SN-SPaCePartiE, grant no. 03F0914E, DAM-SN-CoastalFutures, grant no 03F0911F, balt_ADAPT, grant no. 03F0863). Additionally, funding for this work was partly provided by the EU Data Collection Framework (EU Reg. 199/2008 and 2017/1004).
Author information
Authors and Affiliations
Contributions
MM, MAP and PP conceived the study. MM, BI, PK and DM performed most of the literature search to which all co-authors contributed. The first draft of the manuscript was written by MM, BI and PK with input from all co-authors and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The table 1 is revised.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moyano, M., Illing, B., Akimova, A. et al. Caught in the middle: bottom-up and top-down processes impacting recruitment in a small pelagic fish. Rev Fish Biol Fisheries 33, 55–84 (2023). https://doi.org/10.1007/s11160-022-09739-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-022-09739-2