Abstract
The physical/chemical abatement of gas pollutants creates many technical problems, is costly and entails significant environmental impacts. Biological purification of off-gases is a cheap and ecologically safe way of neutralization of gas pollutants. Despite the recent advances, the main technological challenge nowadays is the purification of volatile organic compounds (VOCs) of hydrophobic character due to their low solubility in water. Among all known biological methods of air purification, the most cost-effective biodegradation of hydrophobic VOCs is conducted by biotrickling filters. In this context, fungi have gained an increasing interest in this field based on their ability to biodegrade hydrophobic VOCs. In addition, biotrickling filtration using fungi can support a superior hydrophobic VOC abatement when compared to the bacterial biofilters. This paper aims at reviewing the latest research results concerning biocatalytic activity of fungi and evaluating the possibilities of their practical application in biofiltration systems to remove hydrophobic VOCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Anthropogenic activity introduces different gas pollutants into the environment, the removal of which entails many technical problems and often generates high costs. Recently, social awareness has resulted in more emphasis on ecological aspects in all human activities. Hence, the selection of the optimal method for air pollutant removal should not be based exclusively on financial aspects but also, and more importantly, on the environmental friendliness following green engineering principles. Despite the fact that the techniques of mitigation of gases generating odour nuisance are relatively well-known, the sustainable removal of hydrophobic volatile organic compounds (VOCs) has not been fully tackled. Thus, biological processes for exhaust gas purification have been extensively investigated in the last few decades. Compared to the other off-gas treatment techniques such as chemical absorption, adsorption and incineration, biotechnologies are characterized by their low operation costs, practically unattended operation, low emission of the secondary pollutants and high purification efficiency upon treatment of high volumes of gas with low or medium concentration of VOCs (Jianming et al. 2014; Abraham et al. 2015; Schiavon et al. 2016; Gospodarek et al. 2019b).
The first biological filter was employed in 1893 in England for sewage treatment. Originally, biofilters were constructed using rock or slag as a filtration medium (Metcalf et al. 1979; Chaudhary et al. 2003). In a publication from 1923, H. Bach presented for the first time the concept of controlling odor (hydrogen sulphide) emissions from composting plants and wastewater treatment plants by means of a soil bed (Leson and Winer 1991). Since 1950, biofiltration has been also used for the filtration of off-gases. In the early 1950s, the first successful applications and patents for biofilters were filed in the United States and Germany (Pomeroy 1957). The developed systems were highly effective, unfortunately only for a short time. They were characterized by a simple structure, consisting only of open spaces filled with soil, under which perforated pipes that distributed air were placed. Due to their structure, these systems required a lot of space due to the low specific activity of the soil. Their major disadvantages were susceptibility to cracking, acidification, drying out and uneven air distribution, which resulted from low air permeability through soil layers. Thus, microorganisms have been employed for the removal of VOCs from air for almost 70 years. Similarly, a detailed investigation was conducted on removal of hydrogen sulphide, sulphur dioxide and thiols from air using microorganisms (Gumerman and Carlson 1966; Carlson et al. 1970; Bremner and Banwart 1976; Baltensperger et al. 2008). One of the first applications of fungi in biofiltration was presented in 1982 in the United Kingdom (Wheatley et al. 1982) in order to improve the economy of odour abatement during sewage treatment. In this case study, the filter bed packing of the pilot installation was colonized with filamentous fungi of Fusarium and Geotrichum species. These organisms developed and multiplied strongly on the highly acidic medium, which effectively outcompeted other microorganisms. The plant was used to treat/process the sewage from a production of dairy products. The process was conducted at pH 4–5 with a high biological oxygen demand. In 1977 in Germany, Bohn and Bohn, designed the first soil biofilter for the removal of organic waste gases (Bohn and Bohn 1986). In 1987, they discovered that sorption was not responsible for odor removal by biofiltration, but biodegradation (Detchanamurthy and Gostomski 2012).
S. Ottengrafa in the 1980s extended the use of biofiltration to the treatment of VOCs and hazardous air pollutants (Ottengraf and Oever 1983). Compost was used to effectively remove moderate concentrations of VOCs from the air. Extending biofiltration research has resulted in the development of two new bioreactor configurations for air pollution control, biotrickling filter and bioscrubbers. In biotrickling filter, a nutrient-laden aqueous solution is constantly recirculated through an inert packing material colonized by microorganisms, treating the gas pollutant present in the gas emission pumped co or countercurrently. On the other hand, bioscrubbers are composed of an absorption unit, where gas pollutant absorption occur, coupled with a suspended growth bioreactor for the biodegradation of the absorbed VOCs. In conventional biofilters, natural packing materials are most often used, e.g. peat, compost, wood chips. This type of biofilter often uses unidentified microorganisms that naturally inhabit a given packed bed. Most of these microorganisms are autotrophs. Sometimes the packing material is additionally inoculated with heterotrophic organisms, whose task is to increase the efficiency of the organic gas pollutant degradation process. The stream of gas to be purified is passed through the bed, in which the removal of pollutants takes place. In biotrickling filters, inert beds naturally laking microorganisms and nutrients, are used as packing materials (e.g. polyurethane foam, Pall rings, Rashig rings) (Marycz et al. 2020). Therefore, packed bed material inoculation with selected microorganisms is needed in biotrickling filters. The nutrients, along with the water phase, are continuously recirculated through the biofilter bed. Finally, bioscrubbers are composed of two main units: an absorber and an aerated bioreactor. The first step of the process takes place in the absorber, which is the absorption of pollutants by the recirculating sorbent. The second step takes place in the bioreactor column, where biodegradation of the compounds removed from the air takes place. The microorganisms used in the process are typically activated sludge, so they are suspended in the liquid phase flowing through the device. This method of biofiltration is especially dedicated to the removal of hydrophilic gas pollutants, due to the fact that the water phase governs pollutant removal in bioscrubbers. The development of biotrickling filters and bioscrubbers allowed greater control of process variables such as pH and biofilm thickness, and subsequently gas pollutant abatament can be operated at higher VOC loading rates than with conventional biofilters.
In 1995, Weber and Hartmans (1996) concluded that fungi could play a significant role in the degradation of gas pollutants in biotrickling filter (BTF), which had been usually attributed to bacteria so far. They inoculated two BTFs for toluene removal with different inocula and observed dominant growth of fungi in one BTF over bacteria in the other BTF, although operation conditions were the same for both. Under nutrient limiting conditions, the fungal BTF revealed significantly higher ability to remove toluene (27 g of carbon per m3 per hour versus 13 g of carbon per m3 per h in the bacterial BTF). Figure 1 shows a schematic diagram where the main milestones of the development of biofiltration are depicted, including the use of fungi for biofiltration of hydrophobic VOCs (Yadav et al. 1995).
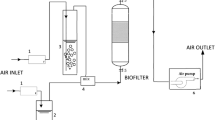
The elimination of gas pollutants in BTFs is the result of a complex combination of various biological and physico-chemical phenomena (Fig. 2). The process of air purification by biological methods consists of the application of microorganisms, most frequently bacteria and fungi, to decompose the VOC to non-toxic or less toxic compounds. While polluted air is passed through the filter bed packing typically upwards, the nutrient medium is recirculated in the column to provide moisture and mineral nutrients for the growth of pollutants-decomposing microorganisms (Cox and Deshusses 1998). During biofiltration, the pollutants contained in air adsorb on a surface of thin biolayer (biofilm), while during biotrickling filtration the gas pollutant must dissolved first in a trickling aqueous solution flowing over this biofilm. Then, the pollutants diffuse into the biofilm, which covers the column’s packing (Figure B). Dissolved pollutants present inside the biofilm are decomposed by microorganisms, which results in the formation of simple reaction products such as CO2, water and biomass following the Eq. (1):
Biofilm—one of the form of microorganims’ growth promote an increase in biofiltration effectiveness up to a point when the amount of biomass is large enough to trigger clogging, which impairs the biofiltration process by creating preferential pathways and anaerobic conditions (Devinny and Ramesh 2005). An advantage of biofilters and BTF is the fact that pollutants are not only transferred to the water phase or the biofilm, but converted into biomass, a compounds less harmful and odorous than their parent pollutants (Revah et al. 2011). Biological processes for off-gas treatment are usually conducted at room temperature and under atmospheric pressure. A drawback of bioprocesses, relatively easy to cope with in BTF, is the sensitivity of microorganisms to non-optimal conditions of temperature, pH or humidity (Kośmider et al. 2012).
The process of air purification consists not only of transferring pollutants from the gas to another phase (aqueous phase or biofilm), but also of removing them: i.e. transformed into less toxic, non-toxic and odorless compounds, converted into biomass or mineralized. The process of decomposition of gas pollutants by microorganisms can take place in two stages: biosorption followed by mineralization, co-metabolic biodegradation or partial biotransformation to intermediates. The first one, so-called biosorption, involves trapping the gas pollutants on the surface of microorganisms’ cells, where a bidirectional exchange occurs: pollutants molecules diffuse into the cells, while enzymes and metabolites travel in the opposite direction (Fig. 3). The second stage, mineralization of the gas pollutants, takes place inside the cells. VOCs are decomposed by microbial enzymes, which results in production of simple molecules. The products of this reaction undergo oxidation after diffusion inside the microorganisms’ cells (Lalanne et al. 2008; Yang et al. 2018).
Adsorption onto the surface of the packing material is often negligible in BTFs due to the inert nature of the bed. Biotrickling filters employ inert beds, which are free of microorganisms and nutrients, for instance polyurethane foam, Pall rings, Rashig rings, expanded clay, ceramic saddles, tri-pack, alginate balls, volcanic rock and perlite (Marycz et al. 2020). In this way, it is possible to inoculate them with selected microorganisms. Nutrients together with water are continuously recirculated through the BTF bed packing, which often has no water retention capabilities. The trickling fluid flowing down the bioreactor is in contact with the biofilm and provides the conditions for control of microbial growth, such as pH, mineral nutrients concentration, conductivity, temperature, etc. Inside the biofilm, pollutant biodegradation occurs due to the catalytic action of bacteria and/or fungi developing in a complex ecosystem. The BTF can be also occupied by predators, for instance protozoa and other higher organisms. Kinetics of pollutants elimination in the biofilm are driven by environmental conditions. BTFs operate in a continuous mode supplying the microorganisms with necessary mineral elements for microbial growth, such as nitrogen, phosphorus, potassium and trace elements. Predators, for example protozoa, nematodes and higher organisms, observed in BTF play an important role in the recycling of key nutrients. Pollutants biodegradation can be accompanied by formation of the final products such as chlorides or sulphates and/or partially oxidized metabolites (i.e. carboxylic acids), which can inhibit biomass growth. The periodic renewal of the trickling solution allows mitigating this product-based inhibition while replenishing essential nutrients. Typically, less than 10% of the carbon present in the pollutants removed is transferred to the trickling liquid and eliminated in this way (Cox et al. 1998).
In recent years, many efforts have been devoted to optimize methods for the abatement of hydrophobic VOCs using microorganisms. Biofiltration is typically cost-effective in the case of exhaust gases with low VOCs concentration (< 3 g/m3) (van Groenestijn and Hesselink 1993). However, conventional biofilters based on compost encounter problems for the elimination of hydrophobic VOCs such as aromatic compounds, alkenes and alkanes. Due to their low solubility in water, these compounds are hardly absorbed by the bacterial biofilms. BTFs inhabited by fungi on an inert material are used to overcome these problems (Cox 1995; Groenestijn et al. 1995). Fungi are more resistant to acidic and dry conditions than bacteria, which is a useful feature upon biofilters maintenance. The hypotheses supporting why fungi exhibit a relatively better performance during the abatement of hydrophobic VOCs are: (i) the aerial mycelium of fungi, which is in direct contact with the gas phase, can absorb hydrophobic VOCs much faster than the flat surfaces of the bacterial biofilm. The aerial mycelium supports high surface area of the biofilm in gas phase, which results in more effective trapping of the hydrophobic VOCs (van Groenestijn et al. 2001): (ii) the extracellular polymeric substance (EPS) can participate in the degradation and sorption of the hydrophobic VOCs (Avalos Ramirez et al. 2012; Han et al. 2020); and finally (iii) the release of surface active substances synthesized by fungi (Ron and Rosenberg 2001b). These biosurfactants can decrease the surface tension and thus facilitate transport of the hydrophobic VOCs to biologically active surfaces.
The topic of fungi applied in biofiltration in recent years has been extensively described by scientists. To date, many aspects of biofiltration involving fungi have been researched and described. However, the reviews published so far indicate new, and therefore unresolved, challenges that remain to be resolved and therefore inspired the authors to write this review. The publication of van Groenestijn and co-workers from 2001, as one of the first, indicated the superiority of the use of fungal over bacterial biofilters for the removal of hydrophobic VOCs (van Groenestijn et al. 2001). The research described in this review identified the important working conditions of fungal biofilters. The review by Kennes and Veiga in 2004 pointed out the dynamic development in the creation of new types of bioreactors, new carrier materials and more efficient biocatalysts (Kennes and Veiga 2004). At that time, the search for efficient fungal biocatalysts, mainly for VOC biofiltration, was presented as a novelty. The publication described novel isolated fungal strains capable of degrading mainly alkylbenzenes. This publication inspired the authors of this review to collect and systematize knowledge about fungal species capable of removing hydrophobic VOCs that have been studied in biotrickling filters in the last 10 years. The review paper of Vergara-Fernández et al. (2018), using conceptual and mathematical models, proved that the abatement efficiency of bacterial biofilters is lower for compounds that are poorly soluble in water compared to fungal biofilters. This review indicated that despite the fact that the main problem in biofilters is the description of the phenomenon of mass and momentum transfer between gas, liquid and biofilm, in order to advance design and optimization of biofilters, it is necessary to understand in detail the relationship between the rate of biodegradation and knowledge of the internal growth of microorganisms in the columns, on various types of packing materials. In a 2018 review by Prenafet-Boldú and co-workers, the the role of melanised hydrocarbonoclastic fungi in biofiltration and biosafety have been discussed was discussed extensively (Prenafeta-Boldú et al. 2018). At that time, there was also no revision of the fungal activity on the removal of VOCs, especially of the hydrophobic nature of their modeling. This review included a detailed description of the phenomena occurring during the biofiltration process (mass, heat and momentum transport) as well as the growth and biodegradation kinetics of bacteria and fungi.
The aim of this paper is the presentation and discussion of the investigation results from the last 10 years on the removal of the hydrophobic VOCs using fungi and their consortia in biofiltration systems. A comparative analysis of fungi and bacteria for the removal of hydrophobic VOCs in biofiltration systems will be carried out. Additionally, the biodegradation potential of fungi not previously exploited in biofiltration will be discussed in term of hydrophobic VOCs removal in biofiltration systems.
2 Comparison of fungi and bacteria in biotrickling filtration
One of the most important parameters in the design of biotrickling filters is the selection of the suitable microorganisms capable of biodegrading the target pollutants. In order to provide the highest biodegradation efficiency, proper operational parameters must be adjusted in every process. The tuning of the parameters influencing on microorganisms growth can result not only in an increase in the VOC biodegradation activity but also cause an increase in microbial growth, which will eventually trigger the process of pollutant removal (under non mass transfer limiting conditions). The microorganisms forming the consortium present in the BTF packing must possess suitable metabolic properties and be capable of cooperation with all microorganisms present in the consortium.
Microorganisms require many macro and micro nutrients to grow and support metabolic activity. Biomass growth and pollutant biodegradation in a bioreactor depend on the number and concentration of nutrients available. These elements are either naturally present in filter bed packings or added to the bed packings when using synthetic or inert materials. Microorganisms are composed of 4 basic elements: carbon, hydrogen, nitrogen and oxygen. In this context, a typical composition of the fungal cells can be described as C4H7N0.6O2, whereas for bacteria the stoichiometric formula is C5H8.3NO1.35 (Shareefdeen et al. 2005).
Microorganisms also require trace elements (Mg, Mn, K, Ca, P, S and Fe) for proper development and operation of enzymes and osmotic equilibrium. Oxygen supply to the microorganisms can be troublesome when treating high loads of highly or moderately soluble VOC or in BTF with thick biofilm layers (Shareefdeen et al. 2005). Regardless of the bioreactor configuration, enzymatic activity of bacteria is often regarded as the dominant factor during biological air purification. However, fungal activity can also play a key role depending on the operational conditions prevailing in the bioreactor. In BTFs operated under non sterile conditions, the packed bed is always inhabited by both bacteria and fungi. Microbial population structure is influenced by the competition among VOC degrading strains, process conditions, technical solutions as well as by microbial contamination since sterile conditions are very difficult to achieve in full scale air purification facilities. Therefore, the composition of microorganisms changes with time and very often without a significant impact on the macroscopic VOC abatement performance. So far, most of the papers published put an emphasis on bacterial population structure in BTFs. Nevertheless, regardless of the conditions in the bioreactor, BTF packing becomes inhabited by other strains that do not belong to the primary microbiota—these could be fungi as well as bacteria feeding on metabolites or cell debris. Thus, the primary microbiota can be substituted with the secondary microbiota. In this context, Maestre et al. (2007) described the change from the dominant bacteria into fungi inside the bed packing in a biofilter treating toluene inoculated only with bacteria (Maestre et al. 2007). The reason underlying this shift in the dominant microbial community was the increased acidification in the biofilter. Interestingly, no decrease in pollutant removal efficiency was observed. On the contrary, a significant improvement in toluene removal was recorded. Due to the fact that fungi are able to survive in much more extreme environmental conditions than bacteria, it is much easier for them to maintain dominant status in the bed packing under long term operation.
Most examples of aromatic hydrocarbons degradation by fungi found in literature are based on a co-metabolism consisting of independent cooperation with the other organisms. One microorganisms typically breaks down the target pollutant into an intermediate metabolite, which becomes available for a second organism that benefits from its partial degradation (Chang et al. 1993). However, there are many studies where fungi were able to use the target organic pollutant as the sole source of carbon and energy. Rybarczyk et al. (2021) reported an efficient removal of cyclohexane and ethanol (95–99%) from air in a BTF inoculated only with the fungal species Candida albicans and Candida subhashi. When comparing the advantages and disadvantages of bacteria and fungi for the removal of gas pollutants in BTFs, scientists have typically focused only on the values of VOC removal efficiency. However, a complete comparative analysis must also take into account the elimination capacity, microbial acclimation time and VOC loading rate and process robustness.
Fungi are eucaryotic organisms that substantially differ in structure and metabolic processes from bacteria, which are the representatives of the procaryotic domain. Table 1 presents a general comparison of morphological and phenotype features of fungi and bacteria (Griffin 1985; Pietarinen et al. 2008).
Today, approximately 70 thousand species of fungi have been described in literature, but it is estimated that their number is much higher in nature (Ławrynowicz 2002). Saprobionts (sporophytes) are an ecological group of fungi, the most frequently presented in papers dealing with gas biofiltration (Thormann and Rice 2007; Gospodarek et al. 2019a). They play a very important role in nature by decomposing organic substrates. Saprobionts convert complex organic substances into simple inorganic compounds and products of their own metabolism, playing a key role on the cycles of the elements in nature. Their specific structure and course of metabolic processes make fungi successful candidates for the removal of VOCs. As above highlighted, the main advantages of fungi over bacteria include:
-
much higher resistance to environmental factors such as temperature, pH and humidity (Kennes and Veiga 2004).
-
ability to survive upon shortage of nutrients. Indeed, when the concentration of chemical substances/nutrients is too low, blocking of enzymes synthesis is not observed.
-
lower sensitivity to the toxic impact of pollutants.
-
some fungi do not need additional time for adaptation, which is typically necessary to start the synthesis of the degradation enzymes in bacteria (Carrera 2010).
In the next sections of the publication (Sects. 2 and 2.1), the authors use the term “biofiltration” to refer to conventional biofiltration and biotrickling filtration processes. This generalization aims at presenting the phenomena which, regardless of the process in which they were described, also take place in both types of the aforementioned biofiltration. In addition, an indisputable advantage of the fungi used in biofilters is their much higher resistance to drying and acidification compared to other organisms (Cox 1995; van Groenestijn et al. 2001). Under mesophilic conditions (15–40 °C), bacteria grow at pH of 5–9, whereas fungi can grow at pH 2–7. The growth as well as the biocatalytic activity of most microorganisms decreases substantially beyond pH 4–8. It is worth noting that almost all microorganisms present in BTFs do not tolerate pH variations higher than 2–3 units (Shareefdeen et al. 2005), whereas microbial activity drops significantly under dry environmental conditions.
Another advantage of fungi in comparison to bacteria is the fact that they possess an aerial mycelium, which significantly increases the surface area of the biofilm in the gas phase, resulting in more effective capture of hydrophobic VOCs (van Groenestijn et al. 2001). All filamentous fungi exhibit branched hypha or numerous hyphae concentrated in one place. Two types of mycelium can be distinguished—substrate (submerged) and aerial (surface) (Fig. 4). The former penetrates the substrate in order to absorbs water and nutrients, whereas the latter develops on the substrate’s surface and is used for respiration and reproduction (Bowman and Free 2006; Ruiz-Herrera 2016). In addition, hydrophobins play a key role in the process of growth and development of the filamentous fungi. These proteins are produced by fungi and participate in the formation of surface structures and hyphae, and their attachment to different types of hydrophobic surfaces. Hydrophobins fulfil these functions as they are produced by fungi on the hydrophobic-hydrophilic surfaces. In BTFs, such surface is established at a liquid-purified gas interface. Based on these properties, fungi can be used in biofilters and biotrickling filters for highly efficient removal of pollutants directly from the gas phase, which allows overcoming VOC mass transfer resistance in the aqueous phase (Wösten et al. 1999; Wösten 2001) and confirms fungi as perfect candidates for removal of the hydrophobic VOCs.
The hydrophobicity of fungal surface can increase proportionally with an increase in the presence of hydrophobic substrates. This phenomenon explains the high efficiency of fungi removing hydrophobic VOCs in biofilters (Vergara-Fernández et al. 2006). However, high pressure drops are recorded when using filamentous fungi, which ultimately entails operational problems such as clogging and channelling of bed packings in biofilters. A solution to these problems can be application of saprophytes during fungal biofiltration. Thus, the addition of higher organisms to biofilters or BTFs prevents from rapid increase in pressure drop and reduces energy consumption for gas circulation. Woertz and co-workers proved that saprophytes in biofilters were relatively easy to maintain during the biofiltration process and could be successfully used to control fungal biomass overgrowth (Woertz et al. 2003). The reduction in the supplementation of some nutrients, such as phosphate and potassium ions or nitrogen, can also limit biomass growth in biofiltration systems (Wübker and Friedrich 1996). Many fungi species also exhibit high potential of surviving during “pollutant surges” and recover full functionality even after few days of starvation (Jin et al. 2007; Rene et al. 2012). Particular attention should be paid to the robustness of VOC purifying biofiltration units on an industrial scale, where transient conditions are relatively common as a result of process shutdown and restart, sudden changes in the VOC loadings. Substrate starvation, which occurs when the bioreactor is deprived of energy and carbon source, is one of the most typical process perturbations. The time of recovery of the biocatalytic activity is influenced by the type of VOC, fasting period, bioreactor configuration and operational parameters.
2.1 Fungal biofilm in biofiltration systems and EPS production
Both bacteria and fungi are able to produce a biofilm with similar functions. However, the publication focuses on a detailed description of only the fungal biofilm due to the topic discussed in the publication. Formation of a biofilm’s structure is a multi-stage process that depends on the properties and structure of the material of the bed packing and on the properties of the microorganisms inhabiting the bioreactor. Figure 5 illustrates the four main stages of biofilm formation. In the first stage, suspended fungal cells attach to the surface of the column’s bed packing. Initially, cells bind onto the substrate via reversible and non-specific interactions (including hydrophobic van der Waals interactions, electrostatic interaction, gravitational forces) (Flemming and Wingender 2010). The extracellular structures of mould fungi, substrate (submerged) mycelia, play an important role in this stage. In the second stage of biofilm formation, a specific reaction takes place between the adhering fungi and the substrate. In this context, the adhesins produced by Candida species have been thoroughly examined and described (Sundstrom 2002; Rapoport et al. 2011). Strong adhesion of the microbial cells to the substrate for a long time creates irreversible connection, which at this stage can be broken only in a mechanical way. The degree of adhesion depends on the physico-chemical properties of the packing, trickling liquid flow rate in BTFs, flow rate of polluted air and the concentration and species of the fungi colonizing the BTF. At this stage of the process, fungal cells produce an extracellular matrix based on extracellular polymeric substance (EPS). Adhesion of cells to the substrate and EPS formation are followed by the third stage of biofilm formation, which involves multiplication and differentiation of the fungal cells.
The factors impacting the extent and rate of biofilm growth include:
-
availability of the elements indispensable for life and growth of fungi.
-
content of nutrients in substrate and trickling liquid.
-
oxygen availability.
-
trickling liquid flow rate.
-
flow rate of supplied polluted air.
-
VOC concentration.
-
pH,
-
ambient temperature,
-
solubility of VOCs in water.
During the last stage of biofilm formation, fungal cells detach from the biofilm structure and via gravitational forces and bulk transport with the trickling liquid, they expand over new surfaces of the BTF bed packing to create a new biofilm. Mature biofilms are a compact, three-dimensional structure composed of a few up to several layers of the fungal cells of the same or different species embedded in EPS. EPS is the main constituent of the biofilm (it corresponds to 90% of the biomass in a mature biofilm, the remaining being fungal cells) responsible for adhesion to the surface of the bed packing and for cohesion inside the film. EPS also forms a scaffolding for a three-dimensional biofilm structure. The main components of EPS and their functions are shown in Table 2. The components of the matrix stabilize the biofilm structure and participate in the formation and maturing of the biofilm, being also a source of nutrients and water. EPS can be also a source of substrate during periods of starvation. The components of the matrix can also protect fungal cells against physical factors, mainly UV radiation.
The knowledge of the structure and functional properties of the biofilm is central to the understanding of its role. Despite the fact that carbohydrates and proteins are considered the main EPS components, biochemical properties of these compounds remain unclear due to their complex structure and unique combinations, and to the fact that each organism can produce different EPS. Moreover, elucidating biofilm’s composition can help explaining the structure–function relations, which can facilitate the design of new strategies to maximize VOC removal in BTFs. This can be the basis of the selection of other species of microorganisms within the consortium, which will work more effectively than pure strains.
EPS are located on or around the surface of the fungal cell and they are considered glycocalyx or slime, which facilitates and accelerates adhesion of fungi to the substrate. EPS contains mainly fungal secretions from the cell’s surface, cellular lysates and hydrolysates and organic substances adsorbed from the environment. EPS is a complex mixture of biomolecules (proteins, polysaccharides, nucleic acids, lipids and other macromolecules), which are released by the microorganisms and maintain microbial aggregates together. Proteins and exopolysaccharides are the key components of the macromolecules, which represents 40–95% of EPS dry matter. EPS was referred to as “a home of biofilm cells” by Flemming et al. (2007), (Flemming et al. 2007), which can be attributed to its three-dimensional (3D) polymer network (comprising over 90% of biofilms). In practice, EPS participates in the transition from reversible to irreversible adhesion of single cells (inhabitation of inert material inside the biofiltration systems) in immobilized but dynamic microbial environment in BTF. This facilitates the formation of compact, three-dimensional polymer networks, which connects and temporarily immobilizes biofilm’s cells. EPS can take part in the degradation and sorption of organic and inorganic compounds and as a barrier to protect cells from hostile environments.
Extracellular polysaccharides, proteins and DNA are strongly hydrated molecules of hydrophilic character, the remaining EPS being composed of hydrophobic molecules. The hydrophobic character of EPS is associated to the acetylene and methyl groups, and lipids combined with polysaccharides, present in the matrix (Neu et al. 1992). Biosurfactants produced by microorganisms in BTFs play an important role during VOC treatment, influencing the surface tension of the trickling solution and thus facilitating gas exchange between the gas and liquid phases. Biosurfactants are surface active substances synthesized by living cells, including yeast (Ron and Rosenberg 2001b), that decrease surface tension, undergo biodegradation and they are generally non-toxic (Neu 1996). Table 3 compiles the main surfactants synthesized by fungal species. In this context, the synthesis of surfactants such as glycolipids and phospholipids is boosted when microorganisms use hydrocarbons as a source of carbon. It was hypothesized that these chemicals are synthesized in order to emulsify the hydrocarbon substrate and to facilitate their transport to the cells (Flemming and Wingender 2010).
3 Application of fungi to biofiltration of hydrophobic compounds
The low aqueous solubility of hydrophobic VOCs is one of the main limitations of biological methods, which entails the need for large gas residence times and bioreactor volumes. A low VOC solubility in water influences gas–liquid or gas-biofilm pollutant transfer, thus significantly limiting the bioavailability of hydrophobic compounds or the possibility of their leaching. In this context, surface active compounds, which are characterized by their ability to change free interphase energy, can decrease the surface tension of the aqueous phase/biofilm and improve the gas–liquid/biofilm mass transfer (Miller et al. 2019). Similarly, the addition of a hydrophilic compound to the BTF can increase the efficiency of hydrophobic VOC removal. This can result in stimulated fungal growth and increased carbon demand of the microbial species inhabiting the biofilm (Cheng et al. 2020), leading to co-metabolism of the hydrophilic compounds in the presence of hydrophobic compounds. However, despite the fact that the synergistic effects of simultaneous removal of hydrophilic and hydrophobic VOCs in biofiltration processes are known (Zhang et al. 2006; Yang et al. 2018), the molecular mechanisms of this improvement have not been identified yet. However, the investigations focused on the mechanism of VOC trapping in the mycelium and in the fungal structure did confirm that fungi could uptake hydrophobic VOCs directly from the gas phase (Krailas et al. 2000). Prenafeta-Boldú and co-workers have published a series of publications in which they demonstrated that black yeast (Capnodiales and Chaetothyriales) can be successfully used for air biofiltration (Prenafeta-Boldú et al. 2008, 2012, 2019; Mayer et al. 2021). These fungi showed a high ability to eliminate VOCs, even those of a hydrophobic nature (e.g. toluene, p-xylene) (Prenafeta-Boldú et al. 2012).
Table 4 presents a review compiling studies of hydrophobic VOCs removal in the biotrickling filtration processes published over the last 10 years. The model fungi employed in these studies were capable of biodegrading aliphatic and aromatic pollutants such as α-pinene, styrene, alkyl benzenes and BTEX (Cox et al. 1996, 1997; Braun-Lüllemann et al. 1997; Shareefdeen et al. 2005).
The overview of the studies using fungi for gas purification of hydrophobic VOCs in biotrickling filtration processes presented in Table 4 shows that the vast majority of studies are focused on the removal of single compounds. The hydrophobic VOCs most frequently removed in BTF inhabited by fungi include toluene, styrene, α–pinene and TCE. The most frequently used trickling liquid is mineral salt medium, often enriched with organic nutrients necessary for the proper functioning and growth of the microorganisms used. EBRT values ranged from several seconds to several minutes (from 15 to 405 s). The different EBRT values resulted, among others, from the variety of dimensions of biofiltration systems, and thus the scale of tests and flowrates of purified gas. Gas chromatography with a flame ionization detector was most often used to evaluate the VOC removal efficiency of the processes. On the other hand, the most popular packing material used in BTFs is polyurethane foam (Moe and Irvine 2000), which is characterised by a high porosity, proper size of the pores to be inhabited by the fungal cells, low density and low pressure drop (Moe and Irvine 2000). Perlite is the second most frequently used packing material in BTFs. Perlite is a naturally occurring amorphous volcanic glass with high thermal and mechanical stability. It is widely used in BTFs due to its non-toxicity, resistance to organic compounds and ease to support the immobilization of microorganisms.
The popular fungal species used in biofiltration studies to abate hydrophobic VOCs were mould (e.g. Fusarium sp.) and yeast (e.g. Candida sp., Cladophialophora sp.). The most efficient fungal species used for the purification of toluene were Exophiala lecanii-corni (RE 95%) (Woertz et al. 2001) and Cladophialophora sp. (RE 99%) (Woertz et al. 2002). Sporothrix variecibatus was the most common species in styrene biodegradation studies, supporting a RE of 95% at EBRTs of 19–77 s (Rene et al. 2010a). In addition, the removal of styrene in the presence of acetone (hydrophilic compound) increased, achieving a RE of 97.5% at an EBRT of 360 s (Rene et al. 2010b). α-pinene removal was carried out with Ophiostoma sp. (RE 95%, EBRT 143 s) (Jin et al. 2006), while TCE abatement in the presence of methanol (hydrophilic compound) has been conducted with a consortium of Fusarium verticillioides and Fusarium solani (max. RE 87.1%, EBRT 9 s) (Quan et al. 2018). Hexane has been effectively removed using Cladophialophora sp. (RE 99%, EBRT 60 s) (Arriaga and Revah 2009) and Candida subhashii was successfully used to remove cyclohexane in the presence of ethanol (hydrophilic compound) (RE 98.9%, EBRT 60 s) (Rybarczyk et al. 2021).
4 Untested fungi with potential to be used for VOC abatement
Table 5 displays fungal species that have not been used in gas phase bioreactors for hydrophobic VOC abatement based on the comprehensive literature search herein conducted. The main fungal candidate for gas biofiltration are mainly white rot fungi with ability to decompose xenobiotics. In fact, many fungal enzymes participating in the decomposition of lignin, including lignin and manganese peroxidase as well as laccase, exhibit a low substrate specificity towards multiple organic compounds of different structure (Cullen and Kersten 1992; Reddy and Mathew 2001). These fungi have been successfully applied in the degradation of toxic, persistent and hardly biodegradable pollutants, which cannot be decomposed by other microorganisms, and of compounds with limited solubility in water (Table 5). For instance, the fungi Trametes versicolor was able to limit significantly the toxicity of chlorinated organic compounds. White rot fungi and some yeast strains can biodegrade highly toxic nitroaromatic compounds under aerobic conditions in a multi-stage process consisting of the reduction of nitro groups and/or aromatic rings. These facts support the potential of the genus Trametes, Trichoderma, Penicillium, Aspergillus, Phanerochaete, Paecilomyces, Scedosporium, Yarrowia, Gymnopilus, Kuehneromyces and Rhizopus to biodegrade hydrophobic VOC in biofilters and BTFs.
5 Summary and future prospects
Despite bacterial biofiltration units are commercially available for air purification, their effectiveness rapidly drops as a result of the low pH, low humidity and nutrient limitations under long term operation. Fungal biofilters and BTFs are considered as more resistant to low humidity and pH and more effective for the removal of hydrophobic VOCs. The most recent literature in the field indicates that many species of fungi, including mold fungi (e.g. Fusarium sp.), yeast (e.g. Candida sp.) and Cladophialophora sp., are able to remove the most common hydrophobic VOCs emitted from industry (α-pinene, styrene, toluene, n-hexane, cyclohexane, TCE) with efficiencies above 90% under optimal design and operational conditions. Fungi can absorb hydrophobic VOCs faster than bacterial biofilm and exhibit unique properties that justify further investigations to fully exploit the potential of these microorganisms.
Compared to conventional fungal biofilters and BTFs, where columns are colonized with one selected species of fungus, an interesting alternative is to use a consortium of different species of fungi to remove hydrophobic VOCs mixtures. In the future, it is necessary to focus on the search for synergistic consortia of fungi, so that individual species of fungi mutually increase their effectiveness in removing hydrophobic VOCs due to cross-feeding interactions or to the excretion of surfactants to the trickling solutions. In this context, understanding the mechanisms inside the fungal cells used in biofiltration systems to remove hydrophobic VOCs and elucidating the biocatalytic properties of fungi and the role of EPS in relation to hydrophobic VOCs will facilitate the selection of the most effective species of fungi, and thus allow the optimization of the biofiltration process. The prospect of using mutagenesis to increase their ability to remove hydrophobic compounds will provide great opportunities for the development of high performance fungal species.
Finally, the use of fungi for the biofiltration of hydrophobic VOCs is in line with the principles of green engineering, which aims at developing processes in a manner conducive to reducing pollution, promoting sustainable development and minimizing the risk to human health and the environment. One of the most promising applications of fungal biofiltration for the principles of green technology is the development of microbial fuel cell technology. This platform is a source of power production, which can be an interesting alternative to the methods used so far, due to the ability of microorganisms to generate electricity while removing pollutants in bioelectrochemical gas-phase reactors.
References
Abraham S, Joslyn S, Suffet IH (2015) Treatment of odor by a seashell biofilter at a wastewater treatment plant. J Air Waste Manag Assoc 65:1217–1228. https://doi.org/10.1080/10962247.2015.1075918
Anasonye F, Winquist E, Räsänen M et al (2015) Bioremediation of TNT contaminated soil with fungi under laboratory and pilot scale conditions. Int Biodeterior Biodegrad 105:7–12
Arellano-García L, Le Borgne S, Revah S (2018) Simultaneous treatment of dimethyl disulfide and hydrogen sulfide in an alkaline biotrickling filter. Chemosphere 191:809–816. https://doi.org/10.1016/j.chemosphere.2017.10.096
Arriaga S, Revah S (2009) Mathematical modeling and simulation of hexane degradation in fungal and bacterial biofilters: effective diffusivity and partition aspects. Can J Civ Eng 36:1919–1925. https://doi.org/10.1139/L09-090 (This article is one of a selection of papers published in this Special Issue on Biological Air Treatment)
Avalos Ramirez A, García-Aguilar BP, Jones JP, Heitz M (2012) Improvement of methane biofiltration by the addition of non-ionic surfactants to biofilters packed with inert materials. Process Biochem 47:76–82. https://doi.org/10.1016/j.procbio.2011.10.007
Baltensperger D, Barbarick K, Roberts C et al (2008) Sulfur: a missing link between soils, crops, and nutrition, vol 50. Agronomy Monograph. Madison
Bhardwaj G, Singh Cameotra S, Kumar Chopra H (2013) Biosurfactants from Fungi: a review petroleum & environmental biotechnology biosurfactants from fungi: a review. J Pet Environ Biotechnol 4:2157–7463. https://doi.org/10.4172/2157-7463.1000160
Bohn H, Bohn R (1986) Gas scrubbing by bio-washers and bio-filters. Pollut Eng 18:34–35
Bowman SM, Free SJ (2006) The structure and synthesis of the fungal cell wall. BioEssays 28:799–808. https://doi.org/10.1002/bies.20441
Braun-Lüllemann A, Majcherczyk A, Hüttermann A (1997) Degradation of styrene by white-rot fungi. Appl Microbiol Biotechnol 47:150–155. https://doi.org/10.1007/s002530050904
Bremner JM, Banwart WL (1976) Sorption of sulfur gases by soils. Soil Biol Biochem 8:79–83
Carlson DA, Leiser CP, Gumerman R (1970) The soil filter: A treatment process for removal of odorous gases. Fed Water Pollut Contr Assoc Rep WP 00883–03
Carrera LV (2010) Two ex situ fungal technologies to treat contaminated soil. In: Academic dissertation, University of Helsinki, Faculty of Agriculture and Foresty, Helsinki
Chang M-K, Voice TC, Criddle CS (1993) Kinetics of competitive inhibition and cometabolism in the biodegradation of benzene, toluene, and p-xylene by two Pseudomonas isolates. Biotechnol Bioeng 41:1057–1065. https://doi.org/10.1002/bit.260411108
Chaudhary DS, Vigneswaran S, Ngo HH et al (2003) Biofilter in water and wastewater treatment. Korean J Chem Eng 20:1054–1065. https://doi.org/10.1007/BF02706936
Cheng Y, Li X, Liu H et al (2020) Effect of presence of hydrophilic volatile organic compounds on removal of hydrophobic n-hexane in biotrickling filters. Chemosphere 252:126490. https://doi.org/10.1016/j.chemosphere.2020.126490
Chheda D, Sorial GA (2017) Evaluation of co-metabolic removal of trichloroethylene in a biotrickling filter under acidic conditions. J Environ Sci (china) 57:54–61. https://doi.org/10.1016/j.jes.2016.12.008
Cox H (1995) Styrene Removal from Waster Gas by the Fungus Exophiala Jeanselmei in a Biofilter. Rijksuniversiteit Groningen, the Netherlands
Cox H, Nguyen T, Deshusses M (1998) Elimination of toluene vapors in biotrickling filters: performance and carbon balances. In: Proceedings of the annual meeting & exhibition of the air & waste management association, pp 14–18
Cox HH, Deshusses MA (1998) Biological waste air treatment in biotrickling filters. Curr Opin Biotechnol 9:256–262. https://doi.org/10.1016/S0958-1669(98)80056-6
Cox HHJ, Magielsen FJ, Doddema HJ, Harder W (1996) Influence of the water content and water activity on styrene degradation by Exophiala jeanselmei in biofilters. Appl Microbiol Biotechnol 45:851–856. https://doi.org/10.1007/s002530050773
Cox HHJ, Moerman RE, van Baalen S et al (1997) Performance of a styrene-degrading biofilter containing the yeast Exophiala jeanselmei. Biotechnol Bioeng 53:259–266. https://doi.org/10.1002/(SICI)1097-0290(19970205)53:3%3c259::AID-BIT3%3e3.0.CO;2-H
Cullen D, Kersten P (1992) Applied molecular genetics of filamentous fungi (Fungal enzymes for lignocellulose degradation). Springer
De S, Malik S, Ghosh A et al (2015) A review on natural surfactants. RSC Adv 5:65757–65767. https://doi.org/10.1039/c5ra11101c
Detchanamurthy S, Gostomski PA (2012) Biofiltration for treating VOCs: an overview. Rev Environ Sci Biotechnol 11(3):231–241. https://doi.org/10.1007/s11157-012-9288-5
Devinny JS, Ramesh J (2005) A phenomenological review of biofilter models. Chem Eng J 113:187–196
Estrada JM, Hernández S, Muñoz R, Revah S (2013) A comparative study of fungal and bacterial biofiltration treating a VOC mixture. J Hazard Mater 250–251:190–197. https://doi.org/10.1016/j.jhazmat.2013.01.064
Ferreira T, Azevedo D, Coelho MA (2009) The crude oil degrading potential of Yarrowia lipolytica. New Biotechnol 25:S80–S81
Flemming HC, Neu TR, Wozniak DJ (2007) The EPS matrix: the “House of Biofilm Cells.” J Bacteriol 189:7945–7947
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633
García-Peña EI, Hernández S, Favela-Torres E et al (2001) Toluene biofiltration by the fungus Scedosporium apiospermum TB1. Biotechnol Bioeng 76:61–69. https://doi.org/10.1002/bit.1026
Gloag ES, Turnbull L, Huang A et al (2013) Self-organization of bacterial biofilms is facilitated by extracellular DNA. Proc Natl Acad Sci USA 110:11541–11546. https://doi.org/10.1073/pnas.1218898110
Gospodarek M, Rybarczyk P, Brillowska-Dąbrowska A, Gębicki J (2019a) The use of various species of fungi in biofiltration of air contaminated with odorous volatile organic compounds. In: E3S web of conferences, vol 100, p 00021. https://doi.org/10.1051/e3sconf/201910000021
Gospodarek M, Rybarczyk P, Szulczyński B, Gębicki J (2019b) Comparative evaluation of selected biological methods for the removal of hydrophilic and hydrophobic odorous VOCs from air. Processes 7:187. https://doi.org/10.3390/pr7040187
Griffin DM (1985) A comparison of the roles of bacteria and fungi. Bacteria in Nature. Springer, pp 221–255
Groenestijn J van, Harkes M, Cox H, Doddema H (1995) Ceramic materials in biofiltration. In: Proceedings USC-TRG conference on biofiltration. Los Angeles, USA
Gumerman R, Carlson DA (1966) Hydrogen sulfide and methyl mercaptan removal with soil columns. In: 21st industrial waste conference, Purdue University. West Lafayette, IN, pp 172–191
Han MF, Hu XR, Wang YC et al (2020) Comparison of separated and combined photodegradation and biofiltration technology for the treatment of volatile organic compounds: a critical review. Crit Rev Environ Sci Technol. https://doi.org/10.1080/10643389.2020.1854566
Husaini A, Roslan H, Hii K et al (2008) Biodegradation of aliphatic hydrocarbon by indigenous fungi isolated from used motor oil contaminated sites. World J Microbiol Biotechnol 24:2789–2797
Ito S, Inoue S (1982) Sophorolipids from torulopsis bombicola: possible relation to alkane uptake. Appl Environ Microbiol 43:1278–1283. https://doi.org/10.1128/aem.43.6.1278-1283.1982
Jianming Y, Wei L, Zhuowei C et al (2014) Dichloromethane removal and microbial variations in a combination of UV pretreatment and biotrickling filtration. J Hazard Mater 268:14–22. https://doi.org/10.1016/j.jhazmat.2013.12.068
Jin Y, Guo L, Veiga MC, Kennes C (2007) Fungal biofiltration of α-pinene: effects of temperature, relative humidity, and transient loads. Biotechnol Bioeng 96:433–443. https://doi.org/10.1002/bit.21123
Jin Y, Veiga MC, Kennes C (2006) Performance optimization of the fungal biodegradation of α-pinene in gas-phase biofilter. Process Biochem 41:1722–1728. https://doi.org/10.1016/j.procbio.2006.03.020
Jorio H, Jin Y, Elmrini H et al (2009) Treatment of VOCs in biofilters inoculated with fungi and microbial consortium. Environ Technol 30:477–485. https://doi.org/10.1080/09593330902778849
Kennes C, Veiga MC (2004) Fungal biocatalysts in the biofiltration of VOC-polluted air. J Biotechnol 113:305–319
Kośmider J, Mazur-Chrzanowska B, Wyszyński B (2012) Odory. Wydawnictwo Naukowe PWN
Krailas S, Pham QT, Amal R et al (2000) Effect of inlet mass loading, water and total bacteria count on methanol elimination using upward flow and downward flow biofilters. J Chem Technol Biotechnol 75:299–305. https://doi.org/10.1002/(SICI)1097-4660(200004)75:4%3c299::AID-JCTB210%3e3.0.CO;2-P
Lalanne F, Malhautier L, Roux J-C, Fanlo J-L (2008) Absorption of a mixture of volatile organic compounds (VOCs) in aqueous solutions of soluble cutting oil. Biores Technol 99:1699–1707. https://doi.org/10.1016/J.BIORTECH.2007.04.006
Ławrynowicz M (2002) Krolestwo grzybow na przelomie tysiacleci. Wiadomości Botaniczne 46:19–25
Leson G, Winer AM (1991) Biofiltration: an innovative air pollution control technology for VOC emissions. J Air Waste Manag Assoc 41:1045–1054. https://doi.org/10.1080/10473289.1991.10466898
Lewandowski Z, Boltz JP (2011) Biofilms in water and wastewater treatment. Treatise on water science. Elsevier, pp 529–570
Li K, Zhou J, Wang L et al (2019) The styrene purification performance of biotrickling filter with toluene-styrene acclimatization under acidic conditions. J Air Waste Manag Assoc 69:944–955. https://doi.org/10.1080/10962247.2019.1604450
Li L, Liu JX (2006) Removal of xylene from off-gas using a bioreactor containing bacteria and fungi. Int Biodeterior Biodegrad 58:60–64. https://doi.org/10.1016/J.IBIOD.2006.07.002
López ME, Rene ER, Malhautier L et al (2013) One-stage biotrickling filter for the removal of a mixture of volatile pollutants from air: performance and microbial community analysis. Bioresour Technol 138:245–252. https://doi.org/10.1016/J.BIORTECH.2013.03.136
Maestre JP, Gamisans X, Gabriel D, Lafuente J (2007) Fungal biofilters for toluene biofiltration: evaluation of the performance with four packing materials under different operating conditions. Chemosphere 67:684–692. https://doi.org/10.1016/J.CHEMOSPHERE.2006.11.004
Marco-Urrea E, Gabarrell X, Sarrà M, Caminal G, Vicent T, Reddy CA (2006) Novel aerobic perchloroethylene degradation by the white-rot fungus Trametes versicolor. Environ Sci Technol 40(24):7796
Marycz M, Brillowska-Dąbrowska A, Gębicki J (2020) Evaluation of immobilization of selected peat-isolated yeast strains of the species Candida albicans and Candida subhashii on the surface of artificial support materials used for biotrickling filtration. Processes 8:801. https://doi.org/10.3390/pr8070801
Mayer VE, de Hoog S, Cristescu SM et al (2021) Volatile organic compounds in the azteca/cecropia ant-plant symbiosis and the role of black fungi. J Fungi 7:836. https://doi.org/10.3390/JOF7100836
Metcalf L, Harrison PE, Tchobanoglous G (1979) Wastewater Engineering: treatment, disposal, and reuse. McGraw-Hill, New York
Miller U, Sówka I, Skrętowicz M (2019) Zastosowanie surfaktantów w biotechnologii środowiska. In: EKO-DOK 11th conference on interdisciplinary problems in environmental protection and engineering. Polanica-Zdrój (Poland)
Moe WM, Irvine RL (2000) Polyurethane foam medium for biofiltration. I: characterization. J Environ Eng 126:815–825. https://doi.org/10.1061/(asce)0733-9372(2000)126:9(815)
Neu TR (1996) Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol Rev 60:151–166
Neu TR, Dengler T, Jann B, Poralla K (1992) Structural studies of an emulsion-stabilizing exopolysaccharide produced by an adhesive, hydrophobic Rhodococcus strain. J Gen Microbiol 138:2531–2537. https://doi.org/10.1099/00221287-138-12-2531
Ottengraf SPP, van den Oever AHC (1983) Kinetics of organic compound removal from waste gases with a biological filter. Biotechnol Bioeng 25:3089–3102. https://doi.org/10.1002/BIT.260251222
Pietarinen VM, Rintala H, Hyvärinen A et al (2008) Quantitative PCR analysis of fungi and bacteria in building materials and comparison to culture-based analysis. J Environ Monit 10:655–663. https://doi.org/10.1039/b716138g
Pomeroy RD (1957) De-odoring of gas streams by the use of micro-biological growths. U.S. Patent No. 2,793,096. U.S. Patent and Trademark Office, Washington, DC
Prenafeta-Boldú FX, de Hoog GS, Summerbell RC (2018) Fungal Communities in Hydrocarbon Degradation. Microbial communities utilizing hydrocarbons and lipids: Members, metagenomics and ecophysiology, handbook of hydrocarbon and lipid microbiology. Springer, Cham
Prenafeta-Boldú FX, Guivernau M, Gallastegui G et al (2012) Fungal/bacterial interactions during the biodegradation of TEX hydrocarbons (toluene, ethylbenzene and p-xylene) in gas biofilters operated under xerophilic conditions. FEMS Microbiol Ecol 80:722–734. https://doi.org/10.1111/J.1574-6941.2012.01344.X
Prenafeta-Boldú FX, Illa J, van Groenestijn JW, Flotats X (2008) Influence of synthetic packing materials on the gas dispersion and biodegradation kinetics in fungal air biofilters. Appl Microbiol Biotechnol 79(2):319–327. https://doi.org/10.1007/S00253-008-1433-2
Prenafeta-Boldú FX, Roca N, Villatoro C et al (2019) Prospective application of melanized fungi for the biofiltration of indoor air in closed bioregenerative systems. J Hazard Mater 361:1–9. https://doi.org/10.1016/J.JHAZMAT.2018.08.059
Qi B, Moe WM, Kinney KA (2005) Treatment of paint spray booth off-gases in a fungal biofilter. J Environ Eng 131:180–189. https://doi.org/10.1061/(ASCE)0733-9372(2005)131:2(180)
Quan Y, Wu H, Guo C et al (2018) Enhancement of TCE removal by a static magnetic field in a fungal biotrickling filter. Bioresour Technol 259:365–372. https://doi.org/10.1016/J.BIORTECH.2018.03.031
Raboni M, Torretta V, Viotti P (2017) Treatment of airborne BTEX by a two-stage biotrickling filter and biofilter, exploiting selected bacterial and fungal consortia. Int J Environ Sci Technol 14:19–28. https://doi.org/10.1007/s13762-016-1127-8
Rapoport A, Borovikova D, Kokina A et al (2011) Immobilisation of yeast cells on the surface of hydroxyapatite ceramics. Process Biochem 46:665–670. https://doi.org/10.1016/j.procbio.2010.11.009
Reddy CA, Mathew Z (2001) Bioremediation potential of white rot fungi. In: British mycological society symposium series, vol. 23. pp 52–78
Rene ER, López ME, Veiga MC, Kennes C (2010a) Performance of a fungal monolith bioreactor for the removal of styrene from polluted air. Biores Technol 101:2608–2615. https://doi.org/10.1016/J.BIORTECH.2009.10.060
Rene ER, Mohammad BT, Veiga MC, Kennes C (2012) Biodegradation of BTEX in a fungal biofilter: influence of operational parameters, effect of shock-loads and substrate stratification. Bioresour Technol 116:204–213. https://doi.org/10.1016/J.BIORTECH.2011.12.006
Rene ER, Montes M, Veiga MC, Kennes C (2011) Styrene removal from polluted air in one and two-liquid phase biotrickling filter: steady and transient-state performance and pressure drop control. Bioresour Technol 102:6791–6800. https://doi.org/10.1016/J.BIORTECH.2011.04.010
Rene ER, Špačková R, Veiga MC, Kennes C (2010b) Biofiltration of mixtures of gas-phase styrene and acetone with the fungus Sporothrix variecibatus. J Hazard Mater 184:204–214. https://doi.org/10.1016/J.JHAZMAT.2010.08.024
Revah S, Vergara-Fernández A, Hernández S (2011) Fungal biofiltration for the elimination of gaseous pollutants from air. Mycofactories. Bentham Science Publishers Ltds, pp 109–120
Rodrigues L, Banat IM, Teixeira J, Oliveira R (2006) Biosurfactants: potential applications in medicine. J Antimicrob Chemother 57:609–618. https://doi.org/10.1093/jac/dkl024
Ron EZ, Rosenberg E (2001a) Natural roles of biosurfactants. Environ Microbiol 3:229–236
Ron EZ, Rosenberg E (2001b) Natural roles of biosurfactants. Minirev Environ Microbiol 3:229–236. https://doi.org/10.1046/j.1462-2920.2001.00190.x
Ruiz-Herrera J (2016) Fungal cell wall: structure, synthesis, and assembly. CRC Press
Rybarczyk P, Marycz M, Szulczyński B et al (2021) Removal of cyclohexane and ethanol from air in biotrickling filters inoculated with Candida albicans and Candida subhashii. Arch Environ Prot. https://doi.org/10.24425/aep.2021.136445
Schiavon M, Ragazzi M, Rada EC, Torretta V (2016) Air pollution control through biotrickling filters: a review considering operational aspects and expected performance. Crit Rev Biotechnol 36:1143–1155. https://doi.org/10.3109/07388551.2015.1100586
Shareefdeen Z, Herner B, Singh A (2005) Biotechnology for air pollution control. Springer, Berlin
Siñeriz F, Hommel RK, Kleber H-P (2001) Production of biosurfactants. Encyclopedia of life support systems. Eolls Publishers, Oxford
Singh R, Paul D, Jain RK (2006) Biofilms: implications in bioremediation. Trends Microbiol 14:389–397
Smalyukh II, Butler J, Shrout JD et al (2008) Elasticity-mediated nematiclike bacterial organization in model extracellular DNA matrix. Phys Rev E Stat Nonlinear Soft Matter Phys 78:030701. https://doi.org/10.1103/PhysRevE.78.030701
Sundstrom P (2002) Adhesion in Candida spp. Cell Microbiol 4:461–469. https://doi.org/10.1046/j.1462-5822.2002.00206.x
Thenmozhi R, Arumugam K, Nagasathya A et al (2013) Studies on Mycoremediation of used engine oil contaminated soil samples. Pelagia Res Libr Adv Appl Sci Res 4:110–118
Thormann MN, Rice AV (2007) Fungi from peatlands. Fungal Divers 24:241–299
van Groenestijn JW, Hesselink PGM (1993) Biotechniques for air pollution control. Biodegradation 4:283–301. https://doi.org/10.1007/BF00695975
van Groenestijn JW, van Heiningen WNM, Kraakman NJR (2001) Biofilters based on the action of fungi. Water Sci Technol 44:227–232
Vergara-Fernández A, Revah S, Moreno-Casas P, Scott F (2018) Biofiltration of volatile organic compounds using fungi and its conceptual and mathematical modeling. Biotechnol Adv 36:1079–1093. https://doi.org/10.1016/j.biotechadv.2018.03.008
Vergara-Fernández A, van Haaren B, Revah S (2006) Phase partition of gaseous hexane and surface hydrophobicity of Fusarium solani when grown in liquid and solid media with hexanol and hexane. Biotechnol Lett 28:2011–2017. https://doi.org/10.1007/s10529-006-9186-4
Weber FJ, Hartmans S (1996) Prevention of clogging in a biological trickle-bed reactor removing toluene from contaminated air. Biotechnol Bioeng 50:91–97. https://doi.org/10.1002/(SICI)1097-0290(19960405)50:1%3c91::AID-BIT10%3e3.0.CO;2-A
Wheatley AD, Mitra RI, Hawkes HA (1982) Protein recovery from dairy industry wastes with aerobic biofiltration. J Chem Technol Biotechnol 32:203–212. https://doi.org/10.1002/jctb.5030320125
Woertz JR, Kinney KA, Kraakman NJR et al (2003) Mite growth on fungus under various environmental conditions and its potential application to biofilters. Exp Appl Acarol 27(4):265–276
Woertz JR, Kinney KA, McIntosh NDP, Szaniszlo PJ (2001) Removal of toluene in a vapor-phase bioreactor containing a strain of the dimorphic black yeastExophiala lecanii-corni. Biotechnol Bioeng 75:550–558. https://doi.org/10.1002/bit.10066
van Woertz W, van Heiningen M, Ee J, van Heiningen W, van Eekert M et al (2002) Dynamic bioreactor operation: effects of packing material and mite predation on toluene removal from off-gas. Appl Microbiol Biotechnol 58:690–694. https://doi.org/10.1007/s00253-002-0944-5
Wösten HAB (2001) Hydrophobins: multipurpose proteins. Annu Rev Microbiol 55:625–646. https://doi.org/10.1146/annurev.micro.55.1.625
Wösten HAB, van Wetter M-A, Lugones LG et al (1999) How a fungus escapes the water to grow into the air. Curr Biol 9:85–88. https://doi.org/10.1016/S0960-9822(99)80019-0
Wübker SM, Friedrich CG (1996) Reduction of biomass in a bioscrubber for waste gas treatment by limited supply of phosphate and potassium ions. Appl Microbiol Biotechnol 46:475–480
Yadav JS, Wallace RE, Reddy CA (1995) Mineralization of mono- and dichlorobenzenes and simultaneous degradation of chloro- and methyl-substituted benzenes by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol 61:677–680
Yang C, Qian H, Li X et al (2018) Simultaneous removal of multicomponent VOCs in biofilters. Trends Biotechnol 36:673–685. https://doi.org/10.1016/J.TIBTECH.2018.02.004
Yousefinejad A, Zamir SM, Nosrati M (2019) Fungal elimination of toluene vapor in one- and two-liquid phase biotrickling filters: effects of inlet concentration, operating temperature, and peroxidase enzyme activity. J Environ Manag 251:109554. https://doi.org/10.1016/J.JENVMAN.2019.109554
Zehraoui A, Wendell D, Sorial GA (2014) Treatment of hydrophobic VOCs in trickling bed air biofilter: emphasis on long-term effect of initial alternate use of hydrophilic VOCs and microbial species evolution. J Air Waste Manag Assoc 64:1102–1111. https://doi.org/10.1080/10962247.2014.925522
Zhang Y, Liss SN, Allen DG (2006) The effects of methanol on the biofiltration of dimethyl sulfide in inorganic biofilters. Biotechnol Bioeng 95:734–743. https://doi.org/10.1002/bit.21033
Zhang Y, Liu J, Qin Y et al (2019) Performance and microbial community evolution of toluene degradation using a fungi-based bio-trickling filter. J Hazard Mater 365:642–649. https://doi.org/10.1016/J.JHAZMAT.2018.11.062
Ziganshin AM, Gerlach R, Borch T et al (2007) Production of eight different hydride complexes and nitrite release from 2,4,6-Trinitrotoluene by Yarrowia lipolytica. Appl Environ Microbiol 73:7898–7905. https://doi.org/10.1128/AEM.01296-07
Acknowledgements
The authors are grateful for the support by the project “Integrated Programme of Development of Gdansk University of Technology” No. POWR.03.05.00-00-Z044/17. The support from the regional government of Castilla y León and the EU-FEDER programme (CLU 2017-09 and UIC 315) is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marycz, M., Brillowska-Dąbrowska, A., Muñoz, R. et al. A state of the art review on the use of fungi in biofiltration to remove volatile hydrophobic pollutants. Rev Environ Sci Biotechnol 21, 225–246 (2022). https://doi.org/10.1007/s11157-021-09608-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-021-09608-7