Abstract
Gastrointestinal nutrient sensing via taste receptors may contribute to weight loss, metabolic improvements, and a reduced preference for sweet and fatty foods following bariatric surgery. This review aimed to investigate the effect of bariatric surgery on the expression of oral and post-oral gastrointestinal taste receptors and associations between taste receptor alterations and clinical outcomes of bariatric surgery. A systematic review was conducted to capture data from both human and animal studies on changes in the expression of taste receptors in oral or post-oral gastrointestinal tissue following any type of bariatric surgery. Databases searched included Medline, Embase, Emcare, APA PsychInfo, Cochrane Library, and CINAHL. Two human and 21 animal studies were included. Bariatric surgery alters the quantity of many sweet, umami, and fatty acid taste receptors in the gastrointestinal tract. Changes to the expression of sweet and amino acid receptors occur most often in intestinal segments surgically repositioned more proximally, such as the alimentary limb after gastric bypass. Conversely, changes to fatty acid receptors were observed more frequently in the colon than in the small intestine. Significant heterogeneity in the methodology of included studies limited conclusions regarding the direction of change in taste receptor expression induced by bariatric surgeries. Few studies have investigated associations between taste receptor expression and clinical outcomes of bariatric surgery. As such, future studies should look to investigate the relationship between bariatric surgery-induced changes to gut taste receptor expression and function and the impact of surgery on taste preferences, food palatability, and eating behaviour.
Registration code in PROSPERO: CRD42022313992
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Taste perception serves mainly to identify nutrients and avoid toxins. It is a multifaceted process, comprising sensation of the flavour of a substance as it comes into contact with its corresponding taste receptor, followed by perception of that sensation as enjoyable, unpleasant, or neutral. This process is entwined with other sensory modalities, such as smell, texture, and temperature detection which combine to provide an overall perception of the flavour of a substance. Traditionally, five basic tastes have been described in humans – sweet, sour, bitter, salty, and umami. More recently, research has demonstrated that fats stimulate a different class of receptors in a similar manner [1].
Each basic taste is detected by specific flavour receptors located on taste cell membranes in taste buds of the oral cavity [1]. Three types of mammalian taste cells have been identified. Type I cells are the most abundant, comprising more than half of all cells in each taste bud, and provide glial-like support to other taste cells [2]. Type II taste cells are the main chemosensory cells for the transduction of sweet, umami, fatty, and bitter flavours. Type III taste cells make up just 2–20% of cells in each taste bud and vary according to anatomical position in the oral cavity [1]. Although the specific receptors for sour and salt taste are still uncertain, nerve conduction studies in rodents show that sour flavours are sensed by type III taste cells [3], and salt taste is likely transmitted via either type I glial-like support cells [2] or a subtype of type II taste cells [4]. Each taste receptor subtype is structurally and functionally distinct. Sweet (T1R2, T1R3), bitter (T2Rs), and umami (T1R1, T1R3) taste receptors are G protein-coupled receptors (GPCRs). On lingual taste cells T1R2 and T1R3 heterodimerise to detect sweet flavours, and T1R1 and T1R3 heterodimerise to recognise umami flavours. Twenty-five T2R bitter taste receptors have been discovered in humans, each functioning as a monomer on lingual taste cells to recognise a wide variety of bitter tasting compounds and potential toxins. These GPCRs signal via a common intracellular signalling pathway involving α-gustducin, phospholipase C β2 (PLCβ2), and transient receptor potential cation channel subfamily M member 5 (TRPM5), leading to the release of adenosine triphosphate (ATP) following stimulation by ingested nutrients [1]. Some fatty acid taste receptors (G protein-coupled receptor 40 [GPR40]/ Free fatty acid receptor 1 [FFAR1] in mice and G protein-coupled receptor 120 [GPR120]/ Free fatty acid receptor 5 [FFAR4] in humans) are also GPCRs that trigger TRPM5 activation and intracellular calcium release to exert their effects [5]. Candidate salt [6] and sour [3, 7] receptors are ion channels, and other sweet (sodium-glucose transporter 1 [SGLT1]) and fatty acid (cluster of differentiation 36 [CD36]) receptors are nutrient transporters, where the presence of intracellular tastants triggers intracellular calcium accumulation [8] or transient elevation of ATP [1] and taste cell membrane depolarisation.
Stimulation of all taste receptors on lingual taste cells eventually results in activation of sensory neurons within branches of the facial, glossopharyngeal and vagus cranial nerves [9]. In humans, these nerves transmit flavour signals via the nucleus of the solitary tract to the gustatory cortices in the anterior insula and frontal operculum and mesocorticolimbic regions, such as the ventral tegmental area and nucleus accumbens, resulting in taste perception and the creation of taste preferences, which guide eating behaviours [9]. Lingual taste cells also secrete glucoregulatory and appetite-related hormones, such as glucagon-like peptide-1 (GLP-1), glucagon, ghrelin, cholecystokinin (CCK) and peptide YY (PYY), following stimulation of taste receptors [10]. Although not completely understood, it is thought that these peptides function as autocrine and paracrine signals to modulate the sensitivity of taste perception at the taste bud level [11].
The same taste receptors responsible for lingual taste transduction of bitter (T2Rs), sweet (T1R2 and T1R3), umami (T1R1 and T1R3), and fatty acid (CD36, GPR40/FFAR1 and GPR120/FFAR4) tastants are also found in the gastric, small bowel, and colonic mucosa [12]. While monomers of the T1R class of taste receptors heterodimerise in the oral cavity to detect sweet and umami taste, T1R1, T1R2, and T1R3 exist and function individually in the gastrointestinal tract [12]. Stimulation of these receptors, as well as bitter and fatty acid monomers leads to release of incretins (GLP-1, Glucose-dependent insulinotropic polypeptide [GIP]) and other satiety hormones (PYY, CCK) [13,14,15,16]. Stimulation of sweet receptors also results in the upregulation of glucose transporters SGLT1 and glucose transporter 2 (GLUT2) on neighbouring enterocytes [17,18,19]. Intragastric infusion of fats and sugars can induce preference for different orally coupled flavoured solutions in rodents [20,21,22]. Likewise, intragastric infusion of bitter compounds can induce flavour aversions to orally-coupled substances, even if the oral substance was initially preferred by the animal [23]. Furthermore, unlike wild-type mice, SGLT1 gene knockout mice develop no preference for oral flavours paired with intragastric glucose infusions compared to those paired with water infusions in two-bottle choice tests [24]. In vivo calcium imaging of vagal neurons demonstrates attenuated activity in response to intra-intestinal glucose or fat infusions in SGLT1 knockout and GPR40/120 double knockout mice, respectively [20]. These studies suggest that gut taste receptors play an important role in the development of food preferences.
Bariatric surgery results in sustained weight loss and metabolic improvements [25,26,27]. Alterations of food preferences and taste perception are frequently reported [28,29,30,31]. This is supported by functional MRI studies, which have demonstrated blunting of response in the mesolimbic reward pathway following ingestion of calorie-dense foods in patients who have undergone gastric bypass and sleeve gastrectomy [30,31,32]. Many of the changes occurring after bariatric surgery, including increases in post-prandial release of PYY and GLP-1, changes to vagal signalling [33] and food preferences parallel known or proposed functions of gastrointestinal taste receptors. Furthermore, expression of gut taste receptors is altered in states of metabolic dysfunction such as type 2 diabetes [34, 35] and obesity [36,37,38,39,40] that are ameliorated by bariatric surgery. As such, a potential role for these receptors in metabolic and food preference changes observed after bariatric surgery is plausible.
This review aims to evaluate and synthesise the current literature on the effect of bariatric surgery on the expression of oral and post-oral gastrointestinal taste receptors. Its secondary aim is to explore the association between these taste receptors and clinical outcomes of bariatric surgery.
2 Methods
2.1 Study design
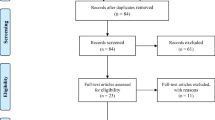
This review was designed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 explanation and elaboration guidelines [41]. A search of electronic databases was conducted in March 2022. Duplicate publications were excluded using Endnote electronic reference manager [42]. Screening was conducted by two independent reviewers using Covidence [43]. Disputes regarding inclusion of studies were resolved by consensus. The reference lists of relevant review articles were searched for potential additional studies. Data was extracted by a single reviewer. Due to the heterogeneity of the included studies conduction of a meta-analysis was not possible.
2.2 Search strategy
A search of Medline (Ovid, 1946–2022), Embase (Ovid, 1974–2022), Emcare (Ovid 1995–2022), APA PsychInfo (Ovid, 1806–2022), Cochrane Library, and CINAHL (EBSCOhost) electronic databases was performed on 10th of March 2022 using a combination of keywords and MeSH terms (Table 1). No date or language limits were applied. Online software ‘Polyglot’ was used to translate search syntax across databases [44].
The publication of research identifying the additional function of the glucose sensor SGLT1 as a sweet taste receptor occurred during the data extraction phase. A supplementary search of the aforementioned databases was conducted on the 25th of May 2022 with the following search terms added to the strategy detailed in Table 1: “SGLT*” or “glucose absor*” or “GLUT*” or “glucose transport*”.
2.3 Eligibility criteria
Human or animal English-language studies were eligible for inclusion. No date restriction was applied.
Inclusion criteria:
-
Full text available in English
-
Any type of bariatric surgery
-
Reports data on tissue analysis of oral or post-oral gastrointestinal taste receptors
-
Reports original data, including randomised controlled trials, cohort studies, case reports and case series.
Exclusion criteria:
-
Analysis of receptors not previously demonstrated to be involved in taste signalling pathways, e.g. bile acid receptors, non-SGLT1 glucose transporters.
-
Artificial tissues – e.g. organoids
-
Uncontrolled studies (those without a non-operated or sham-operated group for comparison).
-
Systematic reviews and meta-analyses, conference abstracts, editorials, and letters-to-the-editor.
2.4 Data collection process
The following information was extracted from the included studies: publication year, study type, population, sample size, type of bariatric surgery performed, method of control, participant age, sex, baseline weight and diabetes status, type and subclass of taste receptor analysed, anatomical location of taste receptor, method of tissue analysis, change (pre-/post-surgery) or difference (surgery vs non-surgery control) in expression or function of taste receptor, time between bariatric surgery and analysis, taste perception or food preference test methods and results, change (pre-/post-surgery) or difference (surgery vs non-surgery control) in weight, fat mass, blood glucose and lipids and circulating gastrointestinal hormone levels.
2.5 Outcomes
The primary outcome is magnitude of change in the tissue expression of oral or post-oral gastrointestinal taste receptors following bariatric surgery.
The secondary outcomes are associations between oral or post-oral gastrointestinal taste receptors and the following clinical outcomes of bariatric surgery; food preference, taste perception, weight loss, fat loss, circulating lipid, glucose, or gastrointestinal hormone levels.
2.6 Risk of bias assessment
The methodological quality and risk of bias of included human studies were assessed using the National Institute of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies [45], and the NIH tool for before-after (pre-post) studies with no control groups [46]. Items #8 (concerning range of exposure) and #10 (repeated exposure assessment) were excluded from the cohort and cross-sectional study assessment due to irrelevance to the review question, as was item #12 (pertaining to statistical analysis of interventions conducted on the group level) on the pre-post study assessment tool.
Animal studies were assessed for internal validity using the SYRCLE risk-of-bias tool, which uses 10 criteria to assess six types of bias: selection, performance, detection, attrition, reporting, and ‘other sources of bias’ [47]. For the purpose of this review ‘other sources’ refers to possible biases resulting from funding sources and conflicts of interest. When assessing selection bias (criterion #3) study groups were considered to be similar at baseline if the species, genotype, age, sex, body weight, and food intake did not significantly differ between groups.
Quality assessments were carried out by two authors independently and discrepancies resolved by consensus.
3 Results
3.1 Study selection and characteristics
The search identified 23 studies for inclusion (Fig. 1), comprising two human studies and 21 animal studies. The two human studies included one longitudinal cohort [48] and one cross-sectional study [49]. The key characteristics are outlined in Table 2. In total, 29 participants underwent Roux-en-Y gastric bypass (RYGB) [48, 49] and 10 participants underwent laparoscopic adjustable gastric banding (LAGB) [48]. Participants had a mean age range of 42 to 52 years, 67% were female and none had diabetes. Time since surgery ranged from four months to 12 years. Taste receptor expression was analysed by quantitative polymerase chain reaction (qPCR) in oral (fungiform papillae) in one study [48], and jejunal/proximal alimentary limb mucosa in the other [49].
Animal studies were conducted in rats (13 studies), mice (seven studies) or both (one study). The bariatric procedure was RYGB in nine studies [50,51,52,53,54,55,56,57,58], duodenal-jejunal bypass (DJB) in four studies [59,60,61,62], sleeve gastrectomy (SG) in seven studies [50, 63,64,65,66,67], and entero-gastric anastomosis (EGA) procedures [35], single-anastomosis duodenal-jejunal bypass (SA-DJB) [68], and ileal interposition (IIP) [69] in one study each. Five studies included analysis of animals with diabetes or insulin resistance [54, 61, 62, 67, 69]. Time between surgery and tissue harvest ranged between 11 days and six months. Taste receptor quantification was carried out using PCR for mRNA analysis in six studies [35, 50, 54, 57, 58, 61], protein analysis techniques (such as Western blotting, immunohistochemistry, or mass spectrometry) in another five [52, 53, 56, 68, 69], or both in 10 studies [51, 55, 59, 60, 62,63,64,65,66,67]. Key characteristics of animal studies are outlined in Table 3.
3.2 Risk of bias
Quality and risk-of-bias assessments are presented in Fig. 2. The overall quality of the human studies was rated as good. The internal validity of the 21 animal studies was limited in each case by lack of reporting on key bias reduction measures, therefore, most items in the risk-of-bias tool were assessed as ‘high risk’ due to omission. Although 12 studies mentioned randomization of group allocation, no study specified the method of randomisation. Similarly, three studies reported using a form of ‘blinding’ to reduce bias but provided minimal detail on the process. Most studies provided sufficient detail regarding pre-intervention characteristics of animals, and all studies adequately addressed the risk of reporting bias (Fig. 2c). The risk of conflicting interest was low for all articles.
3.3 Changes to the gene or protein expression of taste receptors induced by bariatric surgery
3.3.1 Sweet taste receptors
Lingual taste perception of sugars, artificial sweeteners and other sweet compounds occurs primarily through the activation of the T1R2/T1R3 heterodimer [70], although an alternative pathway utilising SGLT1 that is specific for glucose detection also exists [71]. All three receptors have been co-localised to enteroendocrine cells of the gastrointestinal tract [21, 72], where SGLT1 transduces information regarding the presence of glucose and its analogues [21, 24, 73] and T1R3 (with or without T1R2) responds only to artificial sweeteners [21].
Two human [48, 49] and 15 animal studies [50,51,52,53, 55, 57,58,59,60,61,62,63,64,65, 69] examined the effect of bariatric surgery on the sweet taste receptors SGLT1 (14 studies), T1R2 (four studies), and T1R3 (five studies).
SGLT1
One human study reported an increase in basal alimentary limb SGLT1 mRNA expression after RYGB, which was unchanged following a 30-min luminal glucose infusion [49].
Of the animal studies, five reported an increase in SGLT1 mRNA and protein expression in the alimentary limb after RYGB [50,51,52], and DJB [59] and in the interposed ileal segment after IIP [69]. No change to mRNA levels were observed in the alimentary limb of one study [58] or the biliopancreatic limb [50, 58], or colon [57] after RYGB. Two studies reported no change to SGLT1 mRNA in jejunal mucosa after SG [50, 64]. However, one of these studies did find a reduction in jejunal SGLT1 protein expression [64]. Five studies observed a decrease in the mRNA or protein of SGLT1 after bariatric surgery (n = 1 alimentary limb following RYGB, n = 3 alimentary limb following DJB, n = 1 jejunum and ileum following SG) [53, 60,61,62, 65]. Additionally, one study reported a decrease in the abundance of stomach, duodenal, jejunal and ileal SGLT1 mRNA and protein at 2 and 4 weeks after SG, followed by an increased expression above baseline at 8 weeks [63].
T1R2
Two human studies found no change in the mRNA expression of oral [48] or basal proximal alimentary limb [49] T1R2 after RYGB. However, following luminal glucose infusion, T1R2 mRNA expression decreased in the alimentary limb and remained unchanged in non-operated control groups [49]. There was no change to oral T1R2 mRNA expression following LAGB [48].
Two animal studies reported a decrease in the mRNA and protein expression of T1R2 in the alimentary limb [55, 62] and biliopancreatic limbs [55] after RYGB and DJB. No difference in the mRNA or protein levels of T1R2 in the common channel after RYGB was observed [55].
T1R3
No studies examined intestinal T1R3 mRNA or protein expression in humans after bariatric surgery, but one reported no change to oral T1R3 mRNA expression after RYGB and LAGB [48].
Small intestinal T1R3 mRNA and protein expression was decreased following RYGB [55, 58] and DJB [62] in animals. In one of these studies, the reduction was observed only in α-gustducin gene knockout (α-gustducin-KO) mice and not their wild-type counterparts [58]. In the other RYGB study, no change in mRNA expression of T1R3 was found, but T1R3 protein levels were reduced in the alimentary limb when compared to sham-operated control rats [55]. One study reported no change to colonic mRNA expression after RYGB [57].
3.3.2 Amino acid taste receptors
Lingual umami taste transduction occurs primarily through amino acid activation of the T1R1/T1R3 heterodimer [70]. Both T1R1 and T1R3 have been found along the entire length of the gastrointestinal tract individually, however co-localization has previously only been shown in the duodenal mucosa [72]. The T1R3 monomer is shared between the umami and sweet taste pathways and as such, has been discussed in the earlier section on sweet taste receptors.
Lingual T1R1 mRNA expression was not changed in humans after RYGB or LAGB [48]. No study analysed either protein or mRNA expression of T1R1 in the gut after bariatric surgery.
Many other receptors have been implicated in gastrointestinal cell-surface amino acid sensing following ingestion of dietary proteins [74, 75]. Of these, only lysophosphatidic acid receptor 5 (LPAR5, also known as G protein-coupled receptor 92/93 [GPR92/93]) has been investigated for changes following bariatric surgery (two studies) [57, 58]. After RYGB, expression of LPAR5 mRNA increased in the biliopancreatic limb of obese wild-type mice and in the alimentary limb of their α-gustducin-KO counterparts [58]. Colonic LPAR5 mRNA levels were unchanged in mice after RYGB compared to sham-operated, lean, and obese controls [57].
3.3.3 Fatty acid receptors
Taste perception of dietary fats has been proposed as a sixth basic taste [1, 76] but the mechanism by which fatty acid taste perception occurs is not fully elucidated [1]. Several fatty acid receptors and intracellular signalling mediators located on or in oral taste cells or enteroendocrine cells have been proposed as candidate fat taste receptors. These include fatty acid translocase (CD36), free fatty acid receptors 1 – 4 (FFAR1/GPR40, free fatty acid receptor 2 [FFAR2]/ G protein-coupled receptor 43 [GPR43], free fatty acid receptor 3 [FFAR3]/ G protein-coupled receptor 41 [GPR41], FFAR4/GPR120), G protein-coupled receptor 119 [GPR119], G protein-coupled receptor 84 [GPR84], the nuclear receptor peroxisome proliferator-activated receptor-alpha (PPAR- α) and its ligand oleoylethanolamide (OEA) [77,78,79].
Seven animal studies investigated the effect of bariatric surgery on the mRNA or protein expression of gastrointestinal fatty acid receptors [54, 56,57,58, 66,67,68]. FFAR3 mRNA and protein levels were increased in the intestine (location not specified) after SA-DJB [68] and in the colon after RYGB [57]. FFAR2 protein levels were increased following SA-DJB [68] but FFAR2 mRNA levels were unchanged after RYGB [57]. Colonic FFAR1/GPR40, GPR84 and GPR119 mRNA expression was increased post-RYGB [57]. Conversely, one study found a reduction in FFAR3 mRNA expression in the colon of wild-type and α-gustducin-KO mice subjected to RYGB, and a reduction in FFAR2 expression in α-gustducin-KO mice alone [58]. No change to FFAR4 mRNA expression in the alimentary or biliopancreatic limb [58] or colon [57] of RYGB mice was observed.
Post-prandial levels of OEA increased in the alimentary limb and common channel and decreased in the biliopancreatic limb of mice following RYGB [56]. Mice that underwent SG had increased duodenal mRNA expression of CD36, GPR119 and increased intracellular production of OEA but no change to these molecules was found in the jejunum or ileum [66]. PPAR-α mRNA expression remained unchanged in the duodenum, jejunum, and ileum post-SG [66]. SG decreased lingual CD36 mRNA and protein [67] and RYGB decreased jejunal CD36 mRNA [54] in rats.
3.3.4 Alpha-gustducin & other G-protein-coupled receptor (GCPR) intracellular taste signalling machinery
Alpha-gustducin is a primarily taste-specific G-protein alpha subunit responsible for the coupling of sweet, bitter, and umami GPCRs with intracellular second messenger enzyme systems, leading to the opening of cation channels and calcium influx necessary for the release of neurotransmitters (e.g. the purinergic agonist ATP) and peptides (e.g. GLP-1, GIP, PYY) from oral taste cells and enteroendocrine cells [70, 74]. PLCβ2 is an intracellular taste signalling molecule involved in the same calcium influx pathway [70].
One human [48] and one mouse study [35] assessed changes to mRNA expression of intracellular taste signalling molecules. Both studies looked for changes to α-gustducin [35, 48], and one for changes to PLCβ2 [48]. One other study assessed the function of α-gustducin after RYGB using gene knockout mice, which is discussed in earlier sections [58].
Pepino et al. observed a threefold decrease of α-gustducin mRNA and no change to PLCβ2 mRNA in oral fungiform papillae in humans after RYGB and LAGB [48]. In mice, an increase in the abundance of α-gustducin mRNA in the distal jejunum and ileum was reported following EGA [35].
3.3.5 Bitter, salty & sour taste receptors
No study has reported changes to the gene or protein expression of bitter, salty or sour taste receptors in the oral cavity or gastrointestinal tract of humans or animals.
3.4 Association between changes to the gene or protein expression of taste receptors and taste perception and food preference after bariatric surgery
Of the 23 included studies, five assessed changes to taste perception and/or food preference after bariatric surgery alongside changes to the mRNA or protein expression of taste receptors. One was conducted in humans after RYGB and LAGB [48], with the other four assessing food preference and taste detection thresholds in rodents after RYGB [55, 56] and SG [66, 67].
All four studies that assessed differences in fat consumption compared to non-surgical comparators observed a reduced preference for and/or intake of fat after RYGB [48, 56], SG [66, 67], and LAGB [48]. The two studies that examined relationships between changes in fat preference and taste receptor expression found that in mice after SG [66] and RYGB [56], reduced preference for fat is associated with increased intestinal production of intracellular OEA [56] and mediated through PPAR-α activation [56, 66]. Knockout of CD36 attenuated the reduced preference for fat observed in mice after SG whereas GPR119 knockout did not influence eating behaviour [66].
Both studies analysing the impact of bariatric surgery on sweet food intake in humans [48] and rats [55] observed a decreased preference for, and intake of, sugar compared to control groups but neither examined relationships between changes in sweet preference and taste receptor expression. No effect of surgery was found on sweet taste detection thresholds [48].
Two studies assessed changes to intake or detection of umami in humans [48] or rodents [66], two studies assessed salty flavour [48, 55], and one study assessed sour flavour in rats [55], but no changes after bariatric surgery were found, and therefore associations with taste receptor changes were not tested. Details of the studies are outlined in Table 4.
3.5 Association between gene or protein expression of taste receptors and clinical outcomes after bariatric surgery
Studies that investigated an association between expression of gut taste receptors and weight loss, glycaemia and circulating gut hormones after bariatric surgery are summarised in Table 5.
Basal alimentary limb SGLT1 mRNA expression positively correlated with peak post-prandial serum levels of glucose, as well as the non-metabolised glucose analogue 3-O-methyl-D-glucose (3-OMG), in humans after RYGB [49]. The same study found no correlation between plasma concentration of these sugars and T1R2 mRNA expression.
SG reduced weight and improved glucose tolerance in mice with whole body knockout of downstream (PPARα) and upstream (GPR119, CD36) signalling targets of OEA [66]. Circulating GLP-1 levels after a mixed meal test increased similarly after SG in both WT and GPR119KO mice compared with respective sham control animals (58). Due to differences in the effect of sham surgery between α-gustducin gene knockout and wild-type mice, the effects of gustducin-mediated taste receptor signalling on body weight, glucose homeostasis and gut hormone secretion after RYGB are unclear [58].
No studies examined relationship between taste receptor expression and lipid profile after bariatric surgery.
4 Discussion
This is the first systematic review of the effect of bariatric surgery on gastrointestinal taste receptor expression. Overall, the data indicates that changes in mRNA or protein expression of the intracellular taste signalling molecule α-gustducin; sweet taste receptors SGLT1, T1R2, and T1R3; amino acid receptor LPAR5 (GPR92/93); and fatty acid receptors CD36, OEA, FFAR1-3, GPR119, GPR84, occur after all types of bariatric surgery (Fig. 3). Changes to α-gustducin and the sweet and amino acid taste receptors are more commonly reported in intestinal segments that have been surgically repositioned more proximally, such as the alimentary limb after gastric bypass or the interposed ileal segment after ileal interposition surgery. Conversely, changes to fatty acid receptors were more often found in the colon than in the small intestine. Limited data indicate that levels of other taste receptors, including FFAR4, amino acid receptor monomer T1R1; and intracellular taste signalling molecules PLCβ2 and PPAR-α are unaffected by bariatric surgery.
Few studies have examined relationships between taste receptor expression and clinical outcomes of bariatric surgery. Those examining taste preferences have focused on fat. The reduced fat preference observed in mice subjected to SG and RYGB appears to be dependent on CD36 [66] receptors, intracellular intestinal OEA production [56] and PPAR-α activation [56, 66], as disruption of any of these signalling processes by pharmacological blockade or gene knockout negated the effects of bariatric surgery on preference for fat. Conversely, fat receptor GPR119 is not necessary for reduced fat preference after SG [66]. An association between taste receptors and preference for fat has also been reported in non-operated mice, where double-knockout of fat receptors GPR40/FFAR1 and GPR120/FFAR4 prevented the development of fat preference observed in their wild-type or single gene knockout counterparts [20]. Interestingly, this study found no role for CD36 in determining fat preference [20]. A similar gut-brain feeding circuit involving the stimulation of SGLT1 by sugars [20, 24] on CCK-enteroendocrine cells [21] in mice has been reported to be essential in establishing and maintaining the innate mammalian preference sugar over non-nutritive sweeteners. Given that gastrointestinal expression of SGLT1 and CD36, FFAR1-3, GPR119, and GPR84 fatty acid taste receptors change following bariatric surgery, investigation into whether these changes contribute to reduced preferences for sweet and fatty foods after surgery is of interest.
The association between taste receptor expression and post-surgical weight loss is unclear. It is as yet uncertain if changes in taste receptor expression facilitate, result from, or occur independently of changes in food intake, preferences and weight loss. Both oral and post-oral gastrointestinal sweet, fatty acid, umami and bitter taste receptor expression have been shown to correlate with BMI in humans [38, 39, 80, 81], but not all studies agree [34]. No prior study has reported the effect of diet-induced weight loss on taste receptor expression but changes in nutrient intake can acutely alter taste receptor expression. Observational studies in healthy humans have demonstrated that the presence of glucose in the intestinal lumen can acutely alter duodenal expression of sweet taste receptor T1R2 [81]. Short and longer-term consumption of a high-fat diet was associated with reduced expression of oral fatty acid [82] receptors in rodents, whereas humans who followed an 8-week low-fat diet exhibited an increase in oral FFAR4 [83]. After bariatric surgery, most taste receptor expression changes occur in bowel segments newly exposed to incompletely digested nutrients (see Fig. 3) [35, 53, 61, 66], suggesting that changes to taste receptor expression after bariatric surgery are likely to result from altered nutrient exposure. Mechanistic preclinical studies that separate changes in diet quality from changes in body weight would further understanding of the potential relationship between gastrointestinal taste receptors and weight loss. Additional studies assessing food preference changes following manipulation of gut taste receptors would provide insight into the influence of the gut mucosa on eating behaviours.
Association between taste receptor expression and glycaemia following bariatric surgery appears to be taste monomer dependent. Fasting SGLT1 expression was reciprocally associated with glycaemia in humans after RYGB [49], in keeping with the known role of SGLT1 in sugar absorption. The lack of association between basal sweet receptor T1R2 expression and glycaemia after bariatric surgery [49] is in line with prior studies conducted in non-operated individuals with and without diabetes [34].
There is substantial heterogeneity of methods between the 23 included studies, including variations in surgery type, interval between surgery and tissue collection, anatomical location and type of receptor examined, and method of receptor analysis. Hence, the variability in results between studies is not surprising. Different changes to mucosal morphology and expression of sweet taste receptors and glucose transporters observed between RYGB and SG indicates that the intestine adapts differently to the two procedures [50]. Diurnal variation in the expression of oral and post-oral gastrointestinal taste receptors has also been observed in several rodent models [84, 85], yet reporting on the timing of tissue harvest is not standard practice. Furthermore, just as post-operative weight and metabolic benefits of bariatric surgery plateau over time [26, 27], the same pattern may occur for structural adaptations induced by these surgeries [50, 63, 67]. Most of the included studies relied on analysis of mRNA to make conclusions about the effect of bariatric surgery on taste receptors, however this does not capture post-transcriptional changes, such as those reported in three studies that analysed both genes and proteins [51, 55, 64].
Further limitations are that several taste receptor molecules were only investigated by a single study, and many have not been analysed in human tissue. No study has investigated the effect of SG on gastrointestinal taste receptors in humans. By including only studies that utilised tissue analysis, this review may have missed studies examining other changes to taste receptors, including functional changes.
5 Conclusion
This review examines the effect of bariatric surgery on the expression of taste receptors in the oral cavity and along the gastrointestinal tract. While expressional differences in bitter, sweet, fatty and amino acid receptors as well as intracellular taste signalling molecules occur following bariatric surgery, the results are inconsistent. Changes to the gene or protein expression of intracellular α-gustducin, sweet and amino acid receptors occur most often in intestinal segments surgically repositioned more proximally whereas changes to fatty acid receptors were reported more frequently in the colon than in the small intestine. There is a lack of human studies and paucity of data investigating associations between expressional changes and clinical outcomes of bariatric surgery. Understanding the mechanisms that underlie changes in eating behaviour seen in patients after bariatric surgery will facilitate better understanding of the physiology of these surgeries. It may also provide the opportunity to replicate this effect via non-surgical treatments for obesity, such as the development of medications targeting preference for highly palatable foods or the design of effective flavour agonists able to satisfy cravings for sweet or fatty foods without the associated intake in energy.
Data availability
N/A (no original data).
Abbreviations
- GPCR and GPR :
-
G protein-coupled receptor
- PLCβ2 :
-
1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase beta-2
- TRPM5 :
-
Transient receptor potential cation channel subfamily M member 5
- ATP :
-
Adenosine triphosphate
- FFAR :
-
Free fatty acid receptor
- SGLT1 :
-
Sodium-glucose transporter 1
- CD36 :
-
Cluster of differentiation 36, also known as fatty acid translocase
- GLP-1 :
-
Glucagon-like peptide 1
- CCK :
-
Cholecystokinin
- PYY :
-
Peptide YY
- GIP :
-
Glucose-dependent insulinotropic polypeptide, also known as Gastric Inhibitory Peptide
- GLUT2 :
-
Glucose transporter 2
- MRI :
-
Magnetic resonance imaging
- PRISMA :
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- NIH :
-
National Institute of Health
- RYGB :
-
Roux-en-Y gastric bypass
- LAGB :
-
Laparoscopic adjustable gastric banding
- qPCR :
-
Quantitative polymerase chain reaction
- DJB :
-
Duodenal-Jejunal bypass
- SG :
-
Sleeve gastrectomy
- EGA :
-
Entero-gastric anastomosis
- SA-DJB :
-
Single-anastomosis duodenal-Jejunal bypass
- IIP :
-
Ileal interposition
- mRNA :
-
Messenger ribonucleic acid or messenger RNA
- LPAR5 :
-
Lysophosphatidic acid receptor 5
- PPAR- α :
-
Peroxisome proliferator-activated receptor - alpha
- OEA :
-
Oleoylethanolamide
- 3-OMG :
-
3-O-methyl-D-glucose
References
Roper SD, Chaudhari N. Taste buds: cells, signals and synapses. Nat Rev Neurosci. 2017;18(8):485–97. https://doi.org/10.1038/nrn.2017.68.
Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9(1):1. https://doi.org/10.1186/1471-2202-9-1.
Teng B, et al. Cellular and neural responses to sour stimuli require the proton channel Otop1. Curr Biol. 2019;29(21):3647-3656.e5.
Ohmoto M, et al. Sodium-taste cells require Skn-1a for generation and share molecular features with sweet, umami, and bitter taste cells. eNeuro. 2020. https://doi.org/10.1523/ENEURO.0385-20.2020.
Sclafani A, et al. Fat and carbohydrate preferences in mice: the contribution of α-gustducin and Trpm5 taste-signaling proteins. Am J Physiol Regul Integr Comp Physiol. 2007;293(4):R1504–13. https://doi.org/10.1152/ajpregu.00364.2007.
Nomura K, et al. All-electrical Ca2+-independent signal transduction mediates attractive sodium taste in taste buds. Neuron. 2020;106(5):816-829.e6. https://doi.org/10.1016/j.neuron.2020.03.006.
Tu Y-H, et al. An evolutionarily conserved gene family encodes proton-selective ion channels. Science. 2018;359(6379):1047–50.
Gaillard D, et al. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22(5):1458–68.
de Araujo IE, Simon SA. The gustatory cortex and multisensory integration. Int J Obes (Lond). 2009;33(Suppl 2):S34-43. https://doi.org/10.1038/ijo.2009.70.
Dotson CD, Geraedts MCP, Munger SD. Peptide regulators of peripheral taste function. Semin Cell Dev Biol. 2013;24(3):232–9. https://doi.org/10.1016/j.semcdb.2013.01.004.
Shin Y-K, et al. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008;106(1):455–63. https://doi.org/10.1111/j.1471-4159.2008.05397.x.
Depoortere I. Taste receptors of the gut: emerging roles in health and disease. Gut. 2014;63(1):179–90. https://doi.org/10.1136/gutjnl-2013-305112.
Jang HJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104(38):15069–74. https://doi.org/10.1073/pnas.0706890104.
Gerspach AC, et al. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metab. 2011;301(2):E317–25. https://doi.org/10.1152/ajpendo.00077.2011.
Liou AP, et al. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology. 2011;140(3):903–12. https://doi.org/10.1053/j.gastro.2010.10.012.
Kok BP, et al. Intestinal bitter taste receptor activation alters hormone secretion and imparts metabolic benefits. Mol Metab. 2018;16:76–87. https://doi.org/10.1016/j.molmet.2018.07.01.
Margolskee RF, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104(38):15075–80. https://doi.org/10.1073/pnas.07066781.
Moran AW, et al. Expression of Na+/glucose co-transporter 1 (SGLT1) in the intestine of piglets weaned to different concentrations of dietary carbohydrate. Br J Nutr. 2010;104(5):647–55.
Mace OJ, et al. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582(Pt 1):379–92. https://doi.org/10.1113/jphysiol.2007.130906.
Li M, et al. Gut–brain circuits for fat preference. Nature. 2022;610(7933):722–30. https://doi.org/10.1038/s41586-022-05266-z.
Buchanan KL, et al. The preference for sugar over sweetener depends on a gut sensor cell. Nat Neurosci. 2022;25(2):191–200. https://doi.org/10.1038/s41593-021-00982-7.
Kaelberer MM, et al. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361(6408).
Schier LA, Davidson TL, Powley TL. Ongoing ingestive behavior is rapidly suppressed by a preabsorptive, intestinal “bitter taste” cue. Am J Physiol Regul Integr Comp Physiol. 2011;301(5):R1557–68. https://doi.org/10.1152/ajpregu.00344.2011.
Sclafani A, Koepsell H, Ackroff K. SGLT1 sugar transporter/sensor is required for post-oral glucose appetition. Am J Physiol Regul Integr Comp Physiol. 2016;310(7):R631–9. https://doi.org/10.1152/ajpregu.00432.2015.
Jiménez A, et al. Ten-year outcomes after Roux-en-Y gastric bypass and sleeve gastrectomy: an observational nonrandomized cohort study. Surg Obes Relat Dis. 2019;15(3):382–8. https://doi.org/10.1016/j.soard.2019.01.020.
Sjöström L, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304.
Nielsen HJ, et al. Seven-year trajectories of body weight, quality of life and comorbidities following Roux-en-Y gastric bypass and sleeve gastrectomy. Int J Obes (Lond). 2022;46(4):739–49. https://doi.org/10.1038/s41366-021-01028-5.
Ahmed K, et al. Taste changes after bariatric surgery: a systematic review. Obes Surg. 2018;28(10):3321–32. https://doi.org/10.1007/s11695-018-3420-8.
Nielsen MS, et al. Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on food preferences and potential mechanisms involved. Curr Obes Rep. 2019;8(3):292–300. https://doi.org/10.1007/s13679-019-00354-0.
Ochner CN, et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253(3):502–7.
Scholtz S, et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014;63(6):891–902. https://doi.org/10.1136/gutjnl-2013-305008.
Baboumian S, et al. Functional magnetic resonance imaging (fMRI) of neural responses to visual and auditory food stimuli pre and post roux-en-y gastric bypass (RYGB) and sleeve gastrectomy (SG). Neuroscience. 2019;409:290–8. https://doi.org/10.1016/j.neuroscience.2019.01.061.
Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2013;10(10):575–84. https://doi.org/10.1038/nrgastro.2013.119.
Young RL, et al. Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes. 2013;62(10):3532–41. https://doi.org/10.2337/db13-0581.
Le Gléau L, et al. Intestinal alteration of α-gustducin and sweet taste signaling pathway in metabolic diseases is partly rescued after weight loss and diabetes remission. Am J Physiol Endocrinol Metab. 2021;321(3):E417-e432. https://doi.org/10.1152/ajpendo.00071.2021.
Robino A, et al. Taste perception and expression in stomach of bitter taste receptor tas2r38 in obese and lean subjects. Appetite. 2021;166:105595. https://doi.org/10.1016/j.appet.2021.105595.
Latorre R, et al. Expression of the bitter taste receptor, T2R38, in enteroendocrine cells of the colonic mucosa of overweight/obese vs lean subjects. PLoS ONE. 2016;11(2):e0147468. https://doi.org/10.1371/journal.pone.0147468.
Cvijanovic N, et al. Lipid stimulation of fatty acid sensors in the human duodenum: relationship with gastrointestinal hormones, BMI and diet. Int J Obes (Lond). 2017;41(2):233–9. https://doi.org/10.1038/ijo.2016.199.
Little TJ, et al. Characterization of duodenal expression and localization of fatty acid-sensing receptors in humans: relationships with body mass index. Am J Physiol Gastrointest Liver Physiol. 2014;307(10):G958–67. https://doi.org/10.1152/ajpgi.00134.2014.
Yasuo T, et al. Expression of taste signaling elements in jejunal tissue in subjects with obesity. J Oral Biosci. 2022;64(1):155–8. https://doi.org/10.1016/j.job.2021.12.006.
Page MJ, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. https://doi.org/10.1136/bmj.n160.
The EndNote Team. EndNote. Clarivate Analytics: Philadelphia, PA. 2013.
Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org.
Clark JM, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc. 2020;108(2):195–207.
National Heart L, Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies, N.I.o. Health, Editor. 2013.
National Heart L, Blood Institute. Quality assessment tool for before-after (pre-post) studies with no control group, N.I.o. Health, Editor. 2013.
Hooijmans CR, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014. https://doi.org/10.1186/1471-2288-14-43.
Pepino MY, et al. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity. 2014;22(5):E13-20. https://doi.org/10.1002/oby.20649.
Nguyen NQ, et al. Upregulation of intestinal glucose transporters after Roux-en-Y gastric bypass to prevent carbohydrate malabsorption. Obesity. 2014;22(10):2164–71. https://doi.org/10.1002/oby.20829.
Cavin JB, et al. Differences in alimentary glucose absorption and intestinal disposal of blood glucose after Roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology. 2016;150(2):454-64.e9. https://doi.org/10.1053/j.gastro.2015.10.009.
Stearns AT, Balakrishnan A, Tavakkolizadeh A. Impact of Roux-en-Y gastric bypass surgery on rat intestinal glucose transport. Am J Physiol Gastrointest Liver Physiol. 2009;297(5):G950–7. https://doi.org/10.1152/ajpgi.00253.2009.
Pérez-Arana GM, et al. The long-term failure of RYGB surgery in improving T2DM is related to hyperinsulinism. Ann Anat. 2022;240:151855. https://doi.org/10.1016/j.aanat.2021.151855.
Taqi E, et al. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J Pediatr Surg. 2010;45(5):987–95. https://doi.org/10.1016/j.jpedsurg.2010.02.036.
Kaufman S, et al. Roux-en-Y gastric bypass surgery reprograms enterocyte triglyceride metabolism and postprandial secretion in rats. Mol Metab. 2019;23:51–9. https://doi.org/10.1016/j.molmet.2019.03.002.
Bueter M, et al. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav. 2011;104(5):709–21. https://doi.org/10.1016/j.physbeh.2011.07.025.
Hankir MK, et al. Gastric bypass surgery recruits a gut PPAR-alpha-striatal D1R pathway to reduce fat appetite in obese rats. Cell Metab. 2017;25(2):335–44. https://doi.org/10.1016/j.cmet.2016.12.006.
Peiris M, et al. Effects of obesity and gastric bypass surgery on nutrient sensors, endocrine cells, and mucosal innervation of the mouse colon. Nutrients. 2018;10(10):17. https://doi.org/10.3390/nu10101529.
Steensels S, et al. The role of nutrient sensing in the metabolic changes after gastric bypass surgery. J Endocrinol. 2017;232(3):363–76. https://doi.org/10.1530/JOE-16-0541.
Kim M, et al. Changes in glucose transporters, gluconeogenesis, and circadian clock after duodenal-jejunal bypass surgery. Obes Surg. 2015;25(4):635–41. https://doi.org/10.1007/s11695-014-1434-4.
Yan S, et al. Reduction of intestinal electrogenic glucose absorption after duodenojejunal bypass in a mouse model. Obes Surg. 2013;23(9):1361–9. https://doi.org/10.1007/s11695-013-0954-7.
Jurowich CF, et al. Duodenal-jejunal bypass improves glycemia and decreases SGLT1-mediated glucose absorption in rats with streptozotocin-induced type 2 diabetes. Ann Surg. 2013;258(1):89–97. https://doi.org/10.1097/SLA.0b013e3182890311.
Jiang B, et al. Role of proximal intestinal glucose sensing and metabolism in the blood glucose control in type 2 diabetic rats after duodenal jejunal bypass surgery. Obes Surg. 2022;32(4):1119–29. https://doi.org/10.1007/s11695-021-05871-3.
Xia J, et al. Residual Gastric Dilatation Interferes with Metabolic Improvements Following Sleeve Gastrectomy by Upregulating the Expression of Sodium-Glucose Cotransporter-1. Obes Surg. 2019;29(10):3324–33. https://doi.org/10.1007/s11695-019-03997-z.
Du J, et al. Intestinal glucose absorption was reduced by vertical sleeve gastrectomy via decreased gastric leptin secretion. Obes Surg. 2018;28(12):3851–61. https://doi.org/10.1007/s11695-018-3351-4.
Ren Y, et al. Sleeve gastrectomy surgery improves glucose metabolism by downregulating the intestinal expression of sodium-glucose cotransporter-3. J Invest Surg. 2022;35(1):14–22. https://doi.org/10.1080/08941939.2020.1810370.
Hutch CR, et al. Oea signaling pathways and the metabolic benefits of vertical sleeve gastrectomy. Ann Surg. 2020;271(3):509–18. https://doi.org/10.1097/SLA.0000000000003093.
Fruhbeck G, et al. High plasma and lingual uroguanylin as potential contributors to changes in food preference after sleeve gastrectomy. Metabolism. 2022;128: 155119. https://doi.org/10.1016/j.metabol.2021.155119.
Yu X, et al. Single-anastomosis duodenal jejunal bypass improve glucose metabolism by regulating gut microbiota and short-chain fatty acids in Goto-Kakisaki rats. Front Microbiol. 2020;11:273. https://doi.org/10.3389/fmicb.2020.00273.
Jurowich CF, et al. Ileal interposition in rats with experimental type 2 like diabetes improves glycemic control independently of glucose absorption. J Diabetes Res. 2015;2015:490365. https://doi.org/10.1155/2015/490365.
Chandrashekar J, et al. The receptors and cells for mammalian taste. Nature. 2006;444(7117):288–94. https://doi.org/10.1038/nature05401.
Breslin PAS, et al. Evidence that human oral glucose detection involves a sweet taste pathway and a glucose transporter pathway. PLoS ONE. 2021;16(10):e0256989. https://doi.org/10.1371/journal.pone.0256989.
Bezençon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32(1):41–9. https://doi.org/10.1093/chemse/bjl034.
Tan H-E, et al. The gut–brain axis mediates sugar preference. Nature. 2020;580(7804):511–6. https://doi.org/10.1038/s41586-020-2199-7.
Reimann F, Tolhurst G, Gribble FM. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012;15(4):421–31. https://doi.org/10.1016/j.cmet.2011.12.019.
Duraffourd C, et al. Mu-opioid receptors and dietary protein stimulate a gut-brain neural circuitry limiting food intake. Cell. 2012;150(2):377–88. https://doi.org/10.1016/j.cell.2012.05.039.
Running CA, Craig BA, Mattes RD. Oleogustus: the unique taste of fat. Chem Senses. 2015;40(7):507–16. https://doi.org/10.1093/chemse/bjv036.
Liu D, et al. Expression of the candidate fat taste receptors in human fungiform papillae and the association with fat taste function. Br J Nutr. 2018;120(1):64–73. https://doi.org/10.1017/S0007114518001265.
Cartoni C, et al. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30(25):8376–82. https://doi.org/10.1523/JNEUROSCI.0496-10.2010.
Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58(5):1058–66. https://doi.org/10.2337/db08-1237.
Archer N, et al. Obesity is associated with altered gene expression in human tastebuds. Int J Obes (Lond). 2019;43(7):1475–84. https://doi.org/10.1038/s41366-018-0303-y.
Nguyen NQ, et al. Accelerated intestinal glucose absorption in morbidly obese humans: relationship to glucose transporters, incretin hormones, and glycemia. J Clin Endocrinol Metab. 2015;100(3):968–76. https://doi.org/10.1210/jc.2014-3144.
Martin C, et al. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS ONE. 2011;6(8):e24014. https://doi.org/10.1371/journal.pone.0024014.
Costanzo A, et al. A low-fat diet up-regulates expression of fatty acid taste receptor gene FFAR4 in fungiform papillae in humans: a co-twin randomised controlled trial. Br J Nutr. 2019;122(11):1212–20. https://doi.org/10.1017/S0007114519002368.
O’Brien P, Hewett R, Corpe C. Sugar sensor genes in the murine gastrointestinal tract display a cephalocaudal axis of expression and a diurnal rhythm. Physiol Genomics. 2018;50(6):448–58. https://doi.org/10.1152/physiolgenomics.00139.2017.
Balakrishnan A, et al. Diurnal rhythmicity in glucose uptake is mediated by temporal periodicity in the expression of the sodium-glucose cotransporter (SGLT1). Surgery. 2008;143(6):813–8. https://doi.org/10.1016/j.surg.2008.03.018.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions Nil specific. RMB holds an Australian Research Council Discovery Early Career Award (190101244). PS is supported by an Investigator Grant from the National Health and Medical Research Council (1178482).
Author information
Authors and Affiliations
Contributions
RW, RMB, PS conceived the topic, RW performed the literature search, RW and LC screened articles for inclusion and conducted the risk of bias assessment, RW extracted data and wrote the first draft, all authors revised the manuscript.
Corresponding author
Ethics declarations
Ethical approval
N/A
Informed consent
N/A
Conflict of interest
PS reports co-authorship of manuscripts with medical writing assistance from Novo Nordisk and Eli Lilly. PS reports an unpaid position with the Obesity Collective (leadership group). The other authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Walmsley, R., Chong, L., Hii, M.W. et al. The effect of bariatric surgery on the expression of gastrointestinal taste receptors: A systematic review. Rev Endocr Metab Disord 25, 421–446 (2024). https://doi.org/10.1007/s11154-023-09865-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-023-09865-7