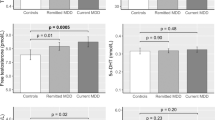
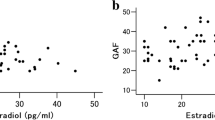
Abstract
Considerable research has shown that testosterone regulates many physiological systems, modulates clinical disorders, and contributes to health outcome. However, studies on the interaction of testosterone levels with depression and the antidepressant effect of testosterone replacement therapy in hypogonadal men with depression have been inconclusive. Current findings indicate that low circulating levels of total testosterone meeting stringent clinical criteria for hypogonadism and testosterone deficiency induced by androgen deprivation therapy are associated with increased risk for depression and current depressive symptoms. The benefits of testosterone replacement therapy in men with major depressive disorder and low testosterone levels in the clinically defined hypogonadal range remain uncertain and require further investigation. Important considerations going forward are that major depressive disorder is a heterogeneous phenotype with depressed individuals differing in inherited polygenic determinants, onset and clinical course, symptom complexes, and comorbidities that contribute to potential multifactorial differences in pathophysiology. Furthermore, polygenic mechanisms are likely to be critical to the biological heterogeneity that influences testosterone-depression interactions. A genetically informed precision medicine approach using genes regulating testosterone levels and androgen receptor sensitivity will likely be essential in gaining critical insight into the role of testosterone in depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Testicular androgens have crucial roles in physiological homeostasis, health outcome, and disease pathophysiology. Testosterone and the more biological active androgen, dihydrotestosterone (DHT), formed by conversion of testosterone by 5α-reductase, act as the primary sex hormones in men regulating male sexual development during puberty and spermatogenesis and sexual function in adulthood [1,2,3] (Fig. 1). Other classical, well-established roles of testosterone include stimulation of erythropoiesis and maintenance of muscular strength and volumetric bone density mass [4, 5] (Fig. 1). Subsequent research, however, has discovered that androgens have more extensive physiological actions regulating cardiovascular, metabolic, hepatic, and immune systems and, importantly, the central nervous system [6,7,8,9,10] (Fig. 1).
Regulation of the hypothalamic-pituitary–gonadal axis, testicular synthesis of androgens, and physiological actions of testosterone resulting from androgen receptor signaling in targeted tissues. The complex, multilevel regulation of the hypothalamic-pituitary–gonadal axis is mediated by stimulatory and inhibitory neurocircuits acting on gonadotropin-releasing hormone (GnRH) neurons in the arcuate/infundibular nucleus and medial preoptic area of the hypothalamus. Testosterone secreted by the testis exerts negative feedback control of hypothalamic GnRH release, while estradiol formed by 5α-reductase conversion of testosterone exerts negative feedback control of anterior pituitary luteinizing hormone (LH) secretion. Synthesis of testosterone and dihydrotestosterone (DHT) by the testis is stimulated by LH activating G protein-coupled LH receptors in Leydig cells. ACTH-stimulated synthesis of DHEA, 5-Adiol, and androstenedione by adrenocortical cells may contribute to testicular synthesis of testosterone and DHT via the “backdoor” pathway, although some studies indicate that DHEA and 5-Adiol secreted by the adrenal cortex may serve as substrates for peripheral conversion of testosterone by androgen receptor-regulated target tissues. Testosterone and DHT secreted by the testis bind to and activate the androgen receptor (AR) expressed in peripheral organs and the central nervous system. The slower genomic actions resulting from classical, canonical androgen receptor signaling involve dissociation of cytosolic AR from heat shock proteins, translocation of AR with chaperones to the nucleus, and then binding of AR and co-regulators to androgen response elements on target genes to activate or repress their expression. In contrast, rapid, non-genomic actions result for membrane androgen receptors signaling via downstream Akt and ERK-MAP kinase pathways. The complex mechanisms governing testosterone hormone action regulate many physiological systems, modulate clinical disorders, and contribute to health outcome. The dotted line indicates an inhibitory action, while the solid line indicates a stimulatory action
The prevalence of major depressive disorder is two-folder higher in women compared to men suggesting that physiological levels of testosterone in the healthy range may reduce the risk of depression [11]. Preclinical research has provided further evidence that androgens may reduce the risk of depression in men due to their antidepressant and neuroprotective actions in the hippocampus, limbic system, and other brain regions regulating mood [12, 13]. Considerable work has shown that low testosterone levels, clinical hypogonadism, pharmacologically induced testosterone deficiency by androgen deprivation therapy, and androgen receptor antagonist treatment are significantly associated with depression in men, although some studies have not observed this effect. An important research question is whether low testosterone levels are a trait biomarker for the depression risk, or a state biomarker associated with a major depressive episode and its severity. Alternatively, however, low testosterone levels may be a result of co-morbid medical conditions associated with depression. The focus of this review will assess the role of testosterone in mood regulation regarding the above important issues.
2 Testosterone levels, hypogonadism, and depression
2.1 Testosterone decline and hypogonadism during aging
In young, healthy men, circulating levels of total testosterone range from 300–1000 ng/dl (10.4–34.7 nmol/L SI units) with 0.5% to 3.0% being free testosterone unbound to sex hormone binding globulin (SHBG) or albumin [1, 2]. The Baltimore Longitudinal Study of Aging has reported that 80% of 60-year-old men and 50% of 80-year-old men exhibit total testosterone levels within the normal range of young men [14, 15]. Other men, however, experience a substantial age-related decline in total testosterone into the clinical hypogonadal range below 280–300 ng/dl (9.7–10.4 nmol/L SI units). Many early cross-sectional studies reported that total testosterone levels in men begin to decline at the age of 40 by a rate of 0.4% per year [15, 16]. Other cross-sectional research found that free testosterone levels decreased more rapidly at a rate of 1.5–2.0% in older men due to the age-dependent upregulation of SHBG [16]. A smaller number of longitudinal studies reported a greater rate of testosterone decline during aging with total testosterone decreasing by 1–2% per year [15, 16].
Although most studies on testosterone decline during aging have involved older men, a recent longitudinal study of young, healthy men (average age 34) found that the age at baseline did not predict changes in the trajectories of testosterone, dihydrotestosterone, androstenedione, and estradiol measured by LC–MS/MS mass spectrometry over a twelve-year period [17]. Furthermore, gonadotropin secretion was upregulated and the testosterone/ luteinizing hormone ratio was decreased indicating declining Leydig cell function despite these men being young. BMI was negatively associated with circulating levels of total and free testosterone, DHT, androstenedione, and estradiol [17].
Research has shown that the age-related decrease in testosterone is mediated by several important mechanisms: (1) impaired luteinizing hormone (LH) receptor signaling via the protein kinase A-cyclic AMP pathway; (2) dysregulation of cholesterol transport and metabolism in mitochondrial due to oxidative stress [18], (3) the attrition of Leydig cells [128]. Furthermore, it is well established that the rate of testosterone decline can be accelerated by modifiable lifestyle factors including obesity and alcohol consumption. Several studies have shown that certain chronic medical disorders, especially type 2 diabetes, may be more important in promoting testosterone decline than increasing age [2, 16, 19, 20]. Importantly, recent research has shown that genetic factors can regulate the trajectory of testosterone during aging [21,22,23].
2.2 Relationship of circulating levels of testosterone and depression
Early studies discovered a significant association of increasing severity of major depressive disorder with low circulating levels of total testosterone in men [24]. Subsequently, observational, cross-sectional, or longitudinal studies reported an inverse relationship of depression scores in men with circulating testosterone levels in the low physiological and hypogonadal ranges, while other studies did not find a relationship of depressive symptoms and testosterone levels [20, 25, 26]. In 1999, the Rancho Bernardo Study reported that lower plasma levels of bioavailable testosterone, calculated using SHBG and albumin, and dihydrotestosterone were associated with higher Beck Depression Inventory (BDI) scores in their large cohort of community-dwelling older men (50 to 89 years) [27]. Total testosterone and estradiol were not significantly associated with depressive symptoms. It is also important to note that none of the men in the Rancho Bernardo Study had testosterone levels in the hypogonadal range. Higher estradiol levels have been reported to be associated with depression in young, obese men. Further investigation is required to elucidate the role of estradiol and its interaction with testosterone in depression especially in older men with hypogonadal testosterone level, which has been difficult to study due in part to mass spectrometry being necessary for specific, sensitive, and quantitative measurement. In addition, the roles of dihydrotestosterone, androstenedione, and other androgenic steroids in depression also warrants further investigation.
2.3 Relationship of testosterone deficiency in hypogonadism and depression
Many clinical symptoms of hypogonadism resemble the symptoms of major depressive disorder. Hypogonadal men frequently experience a depressed mood, anhedonia, fatigue, and cognitive impairment, which are four of the five diagnostic criteria A specified for major depressive disorder in the Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders [25]. In 2018, the Endocrine Society Clinical Practice Guideline established criteria for hypogonadism requiring that two morning serum testosterone levels are below 280–300 ng/dl (9.7–10.4 nmol/L SI units) [1]. When clinical criteria for hypogonadism are used, consistent increases in depressive symptomatology and incidence of clinical depression have been reported in hypogonadal men with confirmed testosterone deficiency compared to eugonadal men with testosterone levels in the normal physiological range. In 2004, a careful study using the Department of Veterans Affairs Healthcare System electronic medical record and a Kaplan–Meier survival analysis reported a two-year incidence of major depressive disorder of 21.7% and a shorter time to the development of depression [OR = 3.5, p = 0.01] in men with an average age of 64.5 years and a stringent diagnosis of hypogonadism defined as total testosterone below 200 ng/dl (6.93 nmol/L SI units) compared to eugonadal men [28]. In 2005, using less stringent cutoff for hypogonadal levels defined as total testosterone below 250 ng/dl (8.67 nmol/L SI units), older, hypogonadal men (average age 69 years) with no history of depression had a higher incidence of a depressive episode (by ICD-9 diagnosis) and a more rapid onset of depression [adjusted HR = 2.1, p = 0.002] over a two-year period compared to eugonadal men [28]. Increasing age and a higher number of co-morbid medical disorders were important factors [28].
In 2006, a Canadian study reported that total and bioavailable testosterone were significantly lower in middle-aged depressed men (40–65 years) who had considerably higher BDI and Hamilton depression scores than men enrolled in the Rancho Bernardo Study [29]. Furthermore, using a logistic regression, this study found that high depression scores were present in 61% of men with hypogonadism compared to only 14% of eugonadal men [29]. The cross-sectional Health in Men Study (HIMS) in Australia reported that the risk of depression increased threefold in men with free testosterone level below 60 pg/ml compared to men with a free testosterone level above 100 pg/ml [30]. These findings emphasize that the degree of testosterone deficiency is important. Likewise, in an adjusted linear regression analysis, the prospective Longitudinal Aging Study Amsterdam observed greater depressive symptoms in men with the lowest quartile of calculated free testosterone compared to men in the highest free testosterone quartile [31]. Furthermore, there was twofold increase in the development of depression [HR = 1.989] in men with free testosterone in the hypogonadal range (< 220 pmol/L SI units; < 63.4 pg/ml) over a ten-year follow-up period [31].
In 2016, the Health in Men Study provided further support for the association of hypogonadism and depression by finding that total testosterone levels below 6.41 nmol/L (185 ng/dl) predicted a high risk of developing incident depression in older men (71–88 years) over a ten-year period after adjusting for age, cardiovascular disorders, and diabetes [HR = 1.86] [32]. Men with normal total testosterone levels had a considerably longer depression-free survival period [32]. This study also reported that low levels of dihydrotestosterone, estradiol, and free testosterone (calculated) did not confer risk for developing incident depression. In addition to being a prospective study, another strength of the HIMS study was measuring total testosterone levels using LC–MS/MS mass spectrometry, which is a critical methodology for accurately measuring hypogonadal testosterone levels [32]. Importantly, a recent investigation of 169,886 male participants (40–69 years) without a history of depression in the prospective UK Biobank study also found men with hypogonadal total testosterone levels (< 6 nmol/L) had a higher five-year incidence of major depressive episode [adjusted OR = 1.60] [33]. The association of major depressive disorder incidence with testosterone levels in the hypogonadal range had the largest effect size among the 57 laboratory tests analyzed in the UK Biobank.
2.4 Hypogonadotropic hypogonadism and depression
A previous study found that young men with congenital hypogonadotropic hypogonadism due to a GnRH deficiency who had very low testosterone levels (78 ng/dl; 2.70 nmol/L SI units) compared to normal controls (483 ng/dl; 16.74 mmol/L SI units) exhibited a high incidence of depression [34]. When hypogonadotropic hypogonadal men were treated with testosterone replacement therapy, their Beck depression score decreased by 90% and was similar to normal male controls [34].
2.5 Meta-analyses of testosterone levels and depression
An earlier meta-analysis of five studies found a significant association of total testosterone levels in the hypogonadal range with Hamilton depression (HAM-D) scores [Z = -3.84; p = 0.0001] [35]. A recent meta-analysis of seven studies involving 1,452 men with mean ages ranging from 36 to 74 years demonstrated that low testosterone levels were significantly associated with major depressive disorder [Z = -2.53; p = 0.012] [36]. These meta-analyses further strengthen the concept that clinical hypogonadism confers a high risk for depression in men.
3 Hypothalamic-pituitary–gonadal axis in depression
3.1 Regulation of the hypothalamic-pituitary–gonadal axis
Dysregulation of the hypothalamic-pituitary–gonadal (HPG) axis has been observed in patients with major depressive episodes. Androgen regulation of the hypothalamic-pituitary–gonadal (HPG) axis is critical for homeostatic regulation of synthesis and secretion of testosterone and the most potent androgen dihydrotestosterone (DHT) by the testis (Fig. 1). Because circulating levels of gonadotropins do not change when pituitary androgen receptors are knocked out in transgenic mice, gonadotrophs in the anterior pituitary do not appear to be a site for testosterone negative feedback [37]. Increasing testosterone levels have been found to inhibit hypothalamic GnRH release via classical negative feedback thereby reducing anterior pituitary secretion of LH and FSH and their stimulation of testosterone steroidogenesis [38]. After research indicated GnRH neurons do not express androgen receptors, kisspeptin and its G protein-coupled receptor KISS1R were discovered as important regulators of GnRH neurons [39]. Testosterone feedback without interacting directly with GnRH neurons targets AR-expressing kisspeptin neurons in the arcuate nucleus of the hypothalamus to negatively regulate pulsatile GnRH release and the HPG axis [38, 40].
In addition to regulating the HPG axis via kisspeptin signaling, testosterone also regulates kisspeptin neurons in the amygdala and hippocampus [39]. Interestingly, kisspeptin has been found to have an antidepressant action possibly by modulating brain serotonergic neurons [41]. Considering that the brain serotonergic neuronal system has a critical role in depression and antidepressant treatment, the interaction of testosterone and kisspeptin neurotransmission may have an unrecognized role in major depressive disorder. Other research has shown that testosterone may exert an antidepressant action by activating androgen receptor MAPK-ERK2 signaling in the hippocampus [12].
3.2 Dysregulation of the hypothalamic-pituitary–gonadal axis and depression
In a circadian study, daytime and nocturnal total testosterone levels and the 24-h mean testosterone secretion were significantly lower in men with severe major depressive episodes based on high Hamilton scores and high 24-h mean cortisol secretion [42]. The role of hypothalamic–pituitary–adrenal hypersecretion observed in severe major depressive episodes and the well-known ability of high cortisol to suppress the hypothalamic-pituitary–gonadal axis in the relationship of testosterone and depression requires further investigation.
Subsequent neuroendocrine research including meta-analyses have found that basal testosterone levels and 24-h testosterone secretion are abnormally low in men with major depressive episodes [25, 36, 43]. Basal secretion of LH and FSH, LH pulse frequency, and GnRH-stimulation gonadotropin secretion by the anterior pituitary are not altered in major depressive disorder indicating that anterior pituitary gonadotropin dysregulation may not contribute to low testosterone levels [36, 43, 44]. A recent meta-analysis of hypothalamic-pituitary–gonadal dysregulation in depression raised the caveat that new LH and FSH assays with greater sensitivity and improved quality control should be used to reassess the role of gonadotropin secretion in depression.
4 Androgen deprivation therapy and depression
4.1 Androgen deprivation therapy and testosterone levels
Androgen deprivation therapy (ADT) is the first line treatment for advanced, metastatic, and recurrent prostate cancer due to its ability to dramatically reduce circulating testosterone. ADT involves treatment with a gonadotrophin-releasing hormone (GnRH) superagonist to desensitize and downregulate pituitary GnRH receptors, thereby depleting testosterone [45, 46]. The result is a profound reduction in circulating levels of testosterone and dihydrotestosterone, by up to 97% without any change in SHBG. Importantly, ADT produces a more severe testosterone deficiency decreasing circulating testosterone to castration levels below 20 ng/dl (0.69 nmol/L SI units), in contrast to the considerably smaller reduction in testosterone levels defining clinical hypogonadism (< 280–300 ng/dl; < 9.7–10.4 nmol/L SI units) [45, 46]. ADT decreases to a lesser extent (~ 40%) the secretion of adrenocortical androgens DHEA, its sulfate metabolite DHEA-S, and androstenedione, which is regulated by ACTH [45] (Fig. 1). ADT results in many adverse physiological effects, far more frequent and intense than occurring in clinical hypogonadism, which includes severe fatigue, increased adiposity and obesity, dyslipidemia, insulin resistance, cardiovascular dysregulation, sarcopenia, osteoporosis and fractures, sexual dysfunction, and increased inflammation [47, 48]. These systemic changes can lead to coronary artery disease, type 2 diabetes, and dyslipidemia, and increase the risk of developing depression [49,50,51].
4.2 Studies of androgen deprivation therapy and depression
The rate of depression is significantly higher in men with prostate cancer compared to cancer-free men [52]. Treatment of prostate cancer with radical prostatectomy or radiation therapy has also been associated with depression [52, 53]. However, androgen deprivation therapy has been shown to have a substantially stronger induction of depression. The association of androgen deprivation therapy and depression represents the most extensively studied psychiatric outcome variable due to its detrimental impact on survivorship [49, 51]. Androgen deprivation therapy has been reported to provoke depressive symptoms and increase the incidence of major depressive episodes in many but not all studies. Since 2000, several small, cross-sectional studies have reported that ADT treatment for 3 to 12 months is associated with significant increases in self-reported depression compared to men with prostate cancer without ADT or healthy controls [50, 54,55,56,57,58,59,60,61]. One early study reported the prevalence of major depressive disorder based on DSM-4 criteria in older men (> 65 years) treated with ADT was 12.8%, which was eightfold higher than the national prevalence rate in men at the same age not receiving ADT [59]. In an Asian cohort, the rate of incident depression over a three-year period was 13.9% in men with prostate cancer treated with ADT who had no prior diagnosis of a depressive disorder [62]. Using a Cox proportional hazard regression analysis, this study reported the risk for depression was significantly higher for ADT compared to no treatment with ADT [adjusted HR = 1.93; p = 0.041]. Other small, cross-sectional prostate cancer studies, however, have found no statistical difference in self-reported depressive symptomatology between ADT-treated men compared to men not receiving ADT [50, 57, 63, 64]. The inconsistent findings on the effect of ADT on mood may have resulted from the cross-section design, insufficient statistical control of variables and biases, lack of statistical power, and other methodological limitations.
Recently, however, three studies with large sample sizes and statistical control of variables have shown a strong association of ADT with a depression diagnosis. A retrospective, observational cohort study (N = 79,930) using the electronic medical record of the Department of Veterans Affairs Healthcare System found that ADT significantly increases the risk for developing a depressive episode over a ten-year period [SHR = 1.50; p < 0.001] using a multivariate competing risks regression model [65]. Using an adjusted Cox proportional hazards analysis and propensity matching and controlling for a past diagnosis of depression, a research group at the Harvard Medical School detected increased risks of new onset depression [AHR = 1.23; p < 0.001] and psychiatric hospitalization [AHR = 1.29; p < 0.001] from androgen deprivation therapy for 6 to 36 months compared to no ADT treatment in men with prostate cancer older than 65 [N = 78,552] from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER)-Medicare-linked database [66]. This study uniquely investigated the time-dependence for adverse effects of ADT on mood demonstrating a dose–response relationship of ADT duration and depression. They also found progressive increases in the cumulative incidence of depression [AHR = 1.37; p < 0.001] and risk of inpatient psychiatric treatment [AHR = 1.47; p < 0.001] with prolonged ADT treatment for 1.0 to 2.5 years. This finding provided evidence for the heightened risk of developing a depressive episode with prolonged ADT treatment [66].
An earlier population-based analysis of the SEER-Medicare database also reported a significantly increased incidence of a depressive disorders in men with prostate cancer after ADT compared to men with prostate cancer not receiving ADT and men without cancer [67]. The observed depressogenic effect of ADT was reduced, however, after adjustment of the Cox proportional hazards regression for a diagnosis of a depressive disorder 12 months before the prostate cancer diagnosis or study entry in addition to other variables including age, ancestry, tumor grade/staging, medical comorbidity, and treatment (radical prostatectomy or radiation therapy) [67]. The findings of these two studies indicate that men with prostate cancer and a history of depression are especially vulnerable to the depressogenic effect of ADT. In 2021, the role of ADT in depression was assessed in a new study of younger men (aged 40–64 years) with nonmetastatic prostate cancer with and without ADT using the TRICARE insurance data and controlling for a past diagnosis of depression [66, 68]. Kaplan–Meier analyses detected that an increasing risk of new onset depression from ADT over a six-year period, while a Cox proportional hazards regression analysis found that ADT was associated with an increased risk of new-onset depression [AHR = 2.07; p < 0.001] [68]. Again, there was a dose–response positive relationship between the duration of ADT treatment and the risk for depression [68].
4.3 Meta-analyses of androgen deprivation therapy and depression
There have been two meta-analyses strongly supporting the relationship of androgen deprivation therapy with depression. In 2017, a meta-analysis that identified 18 independent studies with a total of 168,756 men with prostate cancer confirmed that ADT significantly increases the risk of depression by 41% [RR = 1.41; p < 0.001] using a random effects model [57]. The significant association of ADT with depression held when the meta-analysis was restricted to studies of localized prostate cancer or a clinical diagnosis of a depressive disorder rather than a depressive inventory by a physician or patient self-report. Continuous ADT did not confer an increased depression risk compared to intermittent ADT [57]. In 2020, another meta-analysis across six studies also confirmed that ADT significantly increases the risk of depression [HR = 1.51, p < 0.0002] [51].
4.4 Androgen receptor antagonist, androgen synthesis inhibitor, and depression
A retrospective study using a large male cohort with prostate cancer (N = 30,069) from the NCI’s SEER-Medicare-linked database and the Texas Cancer Registry (TCR)-Medicare-linked database compared the cumulative incidence of depression, defined by ICD-9/10 criteria, in men who were treated with second generation anti-androgen treatment, which included the CYP17 inhibitor abiraterone and an androgen receptor antagonist (bicalutamide, nilutamide, flutamide, enzalutamide, apalutamide, darolutamide) to men treated with only ADT [69]. Using a multivariate Cox proportional hazards analysis and propensity-scored weighting, the risk of incident depression over a two-year period was substantially higher in the second-generation anti-androgen treatment group compared to the ADT group [HR = 2.26; p < 0.001] and the control group without any treatment [HR = 2.15; p < 0.001]. In men with metastatic prostate cancer, second-generation anti-androgen treatment resulted in the highest rate of incident depression [69]. This important new finding indicates inhibiting androgen receptor signaling in brain regions regulating mood generates a stronger depressogenic action than inducing very low testosterone levels with ADT in men with prostate cancer.
5 Testosterone replacement therapy and depression
5.1 Testosterone trials
The Testosterone Trials consisting of seven double-blind, placebo-controlled trials has been the largest investigation to date of the efficacy and benefits of testosterone replacement therapy (TRT) in men older than 65 years who have developed age-related hypogonadism based on strict clinical criteria [15, 70,71,72]. In the Testosterone Trials cohort of hypogonadal men were characterized as having two morning total testosterone levels less than 275 ng/dl (9.53 nmol/L SI units), sexual dysfunction, and diminished physical functioning including low vitality. TRT was confirmed to have the following beneficial effects: (1) libido and sexual activity increased with a lesser improvement in erectile function; (2) hemoglobin levels increased by ~ 1.0 g/L in men with iron-deficiency and chronic anemias; (3) volumetric bone mineral density increased especially in the trabecular bone architecture of lumbar spine vertebrae. It is important to note that the Testosterone Trials found that TRT improved mood and decreased depressive symptoms in hypogonadal men. TRT, however, failed to improve cognitive function and increased coronary artery noncalcified plague volume in coronary arteries by 40 mm3/year [15, 70, 71, 73, 74]. This latter finding was not associated with a greater prevalence of cardiovascular events.
5.2 Testosterone treatment and depression
The mood effect of testosterone treatment has been extensively investigated and meta-analyzed in eugonadal and hypogonadal men with depressive symptoms or major depressive disorder with inconclusive results [20, 26, 35, 50, 75,76,77,78,79]. Three early interventional studies of TRT using testosterone gel or intramuscular testosterone undecanoate in men with hypogonadism based on mean total testosterone levels ranging from 230 to 300 ng/dl (7.97–10.40 SI units) reported a significant reduction in depressive symptoms [80,81,82]. In 2014, a meta-analysis of six studies of testosterone treatment in eugonadal and hypogonadal men, including the above three studies, concluded that TRT improved mood and decreased depressive symptoms in men with low to hypogonadal levels of total testosterone [26].
Randomized, placebo-controlled clinical trials have evaluated the benefit of testosterone treatment in men with major depressive disorder. In 2003, a small RCT study reported that the mean Hamilton score (21.8) in younger men (mean age 46.9 years) with hypogonadism and major depressive disorder refractory to antidepressant medications decreased by ~ 60% when their total testosterone levels were increased from 293 to 789 ng/dl (10.16–27.36 nmol/L SI units) by TRT compared to placebo treatment [83]. Findings from subsequent clinical trials and meta-analyses, however, have reported inconsistent findings with some studies showing an antidepressant effect of TRT and other studies finding no benefit when men with major depressive disorder were treated with testosterone, although the effect of hypogonadal testosterone levels has not always been analyzed [20, 26, 35, 50, 75,76,77,78,79]. Nevertheless, the largest random effects meta-analysis of testosterone treatment in eugonadal or hypogonadal men with depression included 27 randomized controlled trials and found a significant antidepressant effect of TRT compared to placebo [OR = 2.30; p = 0.004] [79]. In addition, a dose–response relationship was observed with the strongest antidepressant effect occurring when men were treated with testosterone doses higher than 500 mg/week [79].
Interestingly, in two randomized, double-blind, placebo-controlled clinical trials completed in 2009, testosterone treatment of men with dysthymic disorder, which is a milder, but persistent depressive disorder characterized by an early, insidious onset and a chronic course, had a stronger antidepressant effect [84, 85]. In the Vitality Trial of the Testosterone Trials, mild depressive symptoms in hypogonadal men measured by the Patient Health Questionnaire PHQ-9 were significantly reduced by 29% (p = 0.004) by TRT compared to 18% decrease by placebo over a nine-month treatment period [86]. Furthermore, meta-analyses have shown that TRT has a more consistent antidepressant effect in men with less severe, subclinical depression [20, 75, 78, 79, 87]. The TRAVERSE trial is now being completed to determine whether testosterone replacement therapy provides significant benefit in clinical disorders including depression. At present, however, the Testosterone Trials and other studies have only found that TRT can be beneficial in men with dysthymic disorder or subsyndrome depression that does not meet criteria for major depressive syndrome. These findings suggest that hypogonadal levels of testosterone dysregulate mood and induce depressive symptoms that can be ameliorated by testosterone treatment, but TRT is unlikely to be an antidepressant treatment for major depressive disorder.
6 Androgen receptor regulation and depression
6.1 Molecular biology of androgen receptor structure
These ubiquitous actions of testosterone and dihydrotestosterone (DHT), the most potent androgen, are signaled by the androgen receptor, AR, (NC-IUPHAR nomenclature: NR3C4), which is a member of the superfamily of nuclear steroid hormone receptors and encoded by the AR gene on the long arm of the X chromosome at Xq11-12. The androgen receptor protein consists of a transcriptional regulation domain at the N-terminus that activates or represses target genes, the highly conserved DNA binding domain with two zinc fingers that bind promoter or enhancer DNA consensus sequences of target genes, a small hinge region, and a ligand binding domain at the C-terminus [88, 89]. Testosterone and DHT binding to the ligand binding domain stimulates the androgen receptor protein to assume an active conformation. Testosterone binds to the androgen receptor with a low nanomolar affinity, while the stronger biological action of DHT is mediated by its two-fold higher affinity and five-fold lower rate of dissociation from the AR compared to testosterone. Androgen receptor signaling exerts important biological actions in the testis, prostate, bone, skeletal muscle, heart, vascular smooth muscle, kidney, pulmonary epithelial cells, bone, adipose tissue, and the central nervous system [89, 90]. In the central nervous system, androgen receptors are highly expressed in the arcuate nucleus and other medial basal region of the hypothalamus, the bed nucleus of the stria terminalis and amygdala in limbic pathway, the hippocampus, and the temporal lobe, which are brain regions regulating mood and cognitive function [91, 92]. Androgen receptor expression has been found to be decreased by 2.7-fold in hypothalamus of men with major depressive disorder compared to male controls [93].
6.2 Canonical and non-canonical androgen receptor signaling
Prior to ligand activation, the androgen receptor is sequestered in the cytoplasm where AR is stabilized by heat shock proteins and associated with cytoskeletal proteins and other chaperones [88, 89, 94]. After binding testosterone or DHT, the cytosolic androgen receptor assumes an active confirmation, dissociates from these cytoplasmic proteins, and translocates to the nucleus where the activated AR dimerizes and functions as a ligand-dependent nuclear transcriptional regulator (Fig. 1). The AR then binds to androgen response elements on androgen target genes to activate or repress their expression [88,89,90]. AR transcriptional regulation is modulated by co-regulators that bind to activated androgen receptors in a ligand-dependent manner to co-activate or co-repress target genes. AR regulation of gene transcription also involves recruitment of transcriptional factors, remodeling of chromatin, and modification of histones.
In addition to the slower genomic actions of the cytosolic AR after translocating to the nucleus, androgen receptors expressed on the cell surface have rapid, non-genomic actions by signaling via downstream calcium, Akt, MAPK-ERK kinase, and protein kinase pathways (Fig. 1), which can regulate synaptic plasticity and have other brain actions [88, 94, 95]. The non-canonical actions of membrane androgen receptors may be coordinated with the canonical actions of androgen receptors in the nucleus. Membrane androgen receptor signaling via non-canonical cascades may be especially important in brain neurons and relevant to antidepressant actions of testosterone by promote cell survival, neurogenesis, synaptic density, and synaptic remodeling in the hippocampus, prefrontal cortex, and other brain regions [96].
6.3 Androgen receptor genetics and depression
Missense mutations in the AR ligand binding result in complete or partial androgen insensitivity syndrome, although mutations in the N-terminal domain encoded by exon 1 have recently been shown to induce resistance to androgen actions [97]. The androgen insensitivity syndrome is the most common genetically driven sex developmental abnormality characterized by a female phenotype in a genetically male 46, XY individual and has reported to increase the risk for depression and be associated with a 36% incidence of depression [98].
Androgen receptor affinity and expression can also be genetically regulated by trinucleotide CAG repeat sequences in exon 1 that vary in length from 9 to 36 repeats [99, 100]. Shorter CAG repeat lengths confer higher affinity and sensitivity of the androgen receptor to testosterone and DHT while longer CAG repeat lengths render the androgen receptor less sensitive to androgens [99, 100]. In men, CAG repeat length is normally distributed with an average of 22 repeats and has been shown to be identical in peripheral leukocytes and brain regions regulating mood and cognitive function [101]. Variation in the AR gene has been associated with male reproductive function, cardiovascular health, prostate cancer, bone density, muscle mass, level of testosterone, and rate of change in testosterone with increasing age [99, 100, 102].
An androgen receptor with higher affinity and sensitivity for testosterone due to shorter CAG repeat length in the presence of low testosterone levels has been associated with depression in men with European or African ancestry [103,104,105]. However, a study using a logistic regression analysis with stratification for AR CAG repeat length found that the risk for depression was significantly lower in men with a highly sensitive androgen receptor due to short CAG repeats if their testosterone levels were high [103]. This latter finding suggests that men with an androgen receptor having higher sensitivity and transcription activity due to shorter CAG repeats is more strongly impacted by higher testosterone levels and will be more responsive to testosterone replacement therapy. The androgen receptor may not have important roles in the susceptibility to depression or the positive response to TRT if the androgen receptor has less sensitivity to testosterone due to longer CAG repeats [104, 106,107,108].
7 Neuronal and molecular mechanisms mediating testosterone and depression
Functional neuroimaging studies (fMRI and PET) have found that testosterone can regulate cerebral blood flow and neuronal activity in the amygdala, hippocampus, and frontal and temporal cortex [109,110,111]. Testosterone can also promote synaptic plasticity and synaptic remodeling in limbic brain neurons expressing the androgen receptor and regulating mood [112,113,114]. Testosterone activated androgen receptor signaling in the hippocampus has been shown to upregulate neurogenesis, which may promote antidepressant responses in depression [115].
In preclinical research, androgen receptor signaling in brain regions regulating mood has been reported to have anti-stress and antidepressant effects [12]. Orchiectomy abolishes this antidepressant action of testosterone-activated brain AR signaling, while a transgenic mouse with a deletion of the androgen receptor gene has been shown to develop depressive-like behavior in response to chronic stress compared to wild-type controls [12, 116]. Other research has found that testosterone promotes an antidepressant response by activating androgen receptor signaling via the MAPK-ERK2 cascade in the hippocampus [12, 117].
Deficient serotonergic neurotransmission and reduced serotonin 5-HT1A and 5-HT1B receptor signaling has an important role in the pathophysiology of major depressive disorder and form the basis of the serotonin hypothesis of depression [118]. Increasing synaptic levels of serotonin with selective serotonin reuptake in inhibitors contributes to antidepressant responses in depression [118]. Testosterone treatment upregulates serotonin transporter expression and increases the firing rate of serotonergic dorsal raphe neurons [119, 120] which has been proposed to promote an antidepressant action. Using PET imaging, a recent study has reported that testosterone regulates hippocampal serotonin 5-HT4 receptors and increases brain serotonergic function [121]. Testosterone can also regulate monoamine oxidase and catechol-o-methyl transferase in amygdala, hippocampus, and other limbic brain areas involved in depression and mediating antidepressant responses [12, 122, 123].
8 Summary, conclusions, and future directions
Current findings indicate that low circulating levels of total testosterone meeting stringent clinical criteria for hypogonadism and testosterone deficiency induced by androgen deprivation therapy are associated with increased risk for depression and current depressive symptoms. Furthermore, the Testosterone Trials and other studies have reported that testosterone replacement therapy may only be beneficial in men with dysthymic disorder or subsyndromal depression that does not meet criteria for major depressive syndrome. These findings suggest that hypogonadal levels of testosterone can dysregulate mood and induce depressive symptoms. The studies reviewed here also suggest that a substantial deficiency in testosterone can cause a depressive-like state that can respond to TRT. At present, there is no clinical justification to use TRT as an antidepressant treatment for major depressive disorder. Therefore, the benefits of testosterone replacement therapy on major depressive disorder in men with clinically defined hypogonadism remains uncertain and will hopefully be elucidated by the TRAVERSE Trial and other ongoing research.
Important considerations are that major depressive disorder is a clinically heterogeneous phenotype with depressed individuals differing in inherited polygenic determinants, onset and clinical course, symptom complexes, and comorbidities that contribute to potential multifactorial differences in pathophysiology. Furthermore, polygenic mechanisms are likely to be critical to the biological heterogeneity that influences testosterone-depression interactions. A recent study has identified certain regulatory variants linked to genetic risk for major depressive disorder in a GWAS, which include hippocampal transcription factors enriched for ZMIZ1, a zinc finger co-activator that increases ligand-dependent transcription of the androgen receptor and promotes androgen receptor sumoylation required for androgen receptor function [124]. Research on male twins has provided heritability estimates of 57–58% for total testosterone [125, 126]. Genome-wide association studies (GWAS) from the UK Biobank and other large cohorts have identified the SNP-based heritability for total testosterone to be ~ 20% and free testosterone to be ~ 15% [21,22,23, 127]. Recent GWAS research has identified significant associations of GCKR, BAIAP2L1, JMJD1C, FKBP4, SERPINA1, SHBG, FAM9B, and other gene variants with total testosterone levels [21,22,23, 127] (Fig. 2). Polygenic scores derived from testosterone GWAS data predict testosterone levels and their association with important phenotypes and clinical disorders. As mentioned earlier, a recent investigation of 169,886 male participants (40–69 years) without a history of depression in the prospective UK Biobank study reported that hypogonadal men with very low total testosterone levels (< 6.0 nmol/L; 173 ng/dl) had high incidence of developing a major depressive episode over a five-year period [adjusted OR = 1.60] [33]. The association of major depressive disorder incidence with testosterone levels in the severe hypogonadal range had the largest effect size among the 57 laboratory tests analyzed in the UK Biobank. Using the UK Biobank genetic database, Mendelian randomization analyses found a beneficial, protective effect of genetically predicted, lifelong free testosterone on depression in men [22]. A genetically informed precision medicine approach using genes regulating testosterone levels and androgen receptor sensitivity will likely provide critical insight into the role of testosterone in depression.
Chromosome idiogram map of gene variants that have significant genome-wide association with testosterone. The localization of testosterone gene variants to specific chromosomes is depicted. Gene variants were identified to have genome-wide significance for regulating testosterone based on GWAS studies of morning total testosterone levels in the UK Biobank and Million Veteran Program [21,22,23, 127]
Abbreviations
- ACADS:
-
Acyl-CoA dehydrogenase short chain
- ACTH:
-
Adrenocorticotropin stimulating hormone
- ANOS1:
-
Anosmin 1
- 5-Adiol:
-
Androstenediol
- Akt:
-
Ak strain transforming protein/Protein kinase B
- ADT:
-
Androgen deprivation therapy
- AR:
-
Androgen receptor
- BAIAP2L1:
-
BAR/IMD domain containing adaptor protein 2 like 1 gene
- BDI:
-
Beck Depression Inventory
- cyclic AMP:
-
Cyclic adenosine monophosphate
- CYP17:
-
Cytochrome P450 17α-hydroxylase/17,20-lyase
- CAG repeats:
-
Cytosine-adenine-guanine repeat sequences
- DHEA:
-
Dehydroepiandrosterone
- DHEA-S:
-
Dehydroepiandrosterone-sulfate
- DHT:
-
Dihydrotestosterone
- FAM9B:
-
Family with sequence similarity 9 member B
- FKBP4:
-
FK506-binding protein 4 gene
- fMRI:
-
Functional magnetic resonance imaging
- HIMS:
-
Health in Men Study
- GWAS:
-
Genome-wide association studies
- GCKR:
-
Glucokinase regulator
- GnRH:
-
Gonadotropin-releasing hormone
- HAM-D:
-
Hamilton depression score
- HLA-DOB2:
-
HLA class II histocompatibility complex, class II, DO beta
- HPG axis:
-
Hypothalamic-pituitary–gonadal axis
- ICD-9:
-
International Classification of Diseases, Ninth Revision
- ICD-10:
-
International Classification of Diseases, Tenth Revision
- JMJD1C:
-
Jumonji domain containing 1C gene
- KISS1R :
-
Kisspeptin receptor 1
- LC–MS/MS:
-
Liquid chromatography with tandem mass spectrometry
- LH:
-
Luteinizing hormone
- MVP:
-
Million Veteran Program
- MAPK-ERK:
-
Mitogen-activated protein kinase/Extracellular -signal-regulated kinase
- SEER:
-
National Cancer Institute’s Surveillance, Epidemiology, and End Results
- NC-IUPHAR:
-
Nomenclature and Standards Committee of the International Union of Basic and Clinical Pharmacology
- PET:
-
Positron emission tomography
- PNPLA3:
-
Patatin like phospholipase domain containing 3
- 5-HT1A:
-
Serotonin 5-hydroxytryptamine receptor 1A
- 5-HT1B :
-
Serotonin 5-hydroxytryptamine receptor 1B
- SERPINA1:
-
Serpin family A member 1 gene
- SHBG:
-
Sex hormone binding globulin
- SLCOB1:
-
Solute carrier organic anion transporter family member 1B1
- TDGF1PB:
-
Teratocarcinoma-derived growth factor 1 pseudogene
- TRT:
-
Testosterone replacement therapy
- TRAVERSE:
-
Testosterone Replacement Therapy for Assessment of Long-term Vascular Events
- UGT2B15:
-
UDP glucuronosyltransferase family 2 member B15
- UK Biobank:
-
United Kingdom Biobank
References
Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715–44.
Matsumoto AM, Anawalt BID. Chapter 19. Testicular disorders. In: Melmed S, Auchus RJ, Goldfine AB, Koenig RJ, Rosen CJ, editors. 14th Edition. Amsterdam: Elsevier; 2019. pp. 668–755.
Wilson JD, Griffin JE, George FW, Leshin M. The endocrine control of male phenotypic development. Aust J Biol Sci. 1983;36:101–28.
Shahani S, Braga-Basaria M, Maggio M, Basaria S. Androgens and erythropoiesis: past and present. J Endocrinol Investig. 2009;32:704–16.
Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:271–7.
Gubbels Bupp MR, Jorgensen TN. Androgen-induced immunosuppression Front Immunol. 2018;9:794.
Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217:R25–45.
Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217:R47–71.
Morford J, Wu S, Mauvais-Jarvis F. The impact of androgen actions in neurons on metabolic health and disease. Mol Cell Endocrinol. 2018;465:92–102.
O’Reilly MW, House PJ, Tomlinson JW. Understanding androgen action in adipose tissue. J Steroid Biochem Mol Biol. 2014;143:277–84.
Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC. Schatzberg AF (2016) Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065.
McHenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Front Neuroendocrinol. 2014;35:42–57.
Williams ES, Mazei-Robison M, Robison AJ. Sex differences in major depressive disorder (MDD) and preclinical animal models for the study of depression. Cold Spring Harb Perspect Biol. 2022;14: a039198.
Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731.
Snyder P. Testosterone treatment of late-onset hypogonadism – benefits and risks. Rev Endocr Metab Disord. 2022; Online ahead of print.
Huhtaniemi IT, Wu FCW. Ageing male (part I): pathophysiology and diagnosis of functional hypogonadism. Best Pract Res Clin Endocrinol Metab. 2022; Online ahead of print.
Banica T, Verroken C, Reyns T, Mohmoud A, T’Sjoen GT, Fiers T, Kaufman J-M, Lapauw B. Early decline of androgen levels in healthy adult men: an effect of aging per se? A prospective cohort study. J Clin Endocrinol Metab. 2021;106:1074–83.
Wang Y, Chen F, Ye L, Zirkin B, Chen H. Steroidogenesis in Leydig cells: effects of aging and environmental factors. Reproduction. 2017;154:R111–22.
Kaufman J-M, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–76.
Kaufman J-M, Lapauw B, Mahmoud A, T’Sjoen G, Huhtaniemi IT. Aging and the male reproductive system. Endocr Rev. 2019;40:906–72.
Fantus RJ, Na R, Wei J, Shi Z, Resurreccion WK, Halpern JA, Franco O, Hayward SW, Isaacs WB, Zheng SL, Xu J, Helfand BT. Genetic susceptibility for low testosterone in men and its implications in biology and screening: data from the UK Biobank. Eur Urol Open Sci. 2021;29:36–46.
Mohammadi-Shemirani P, Chong M, Pigeyre M, Morton RW, Gerstein HC, Pare G. Effects of lifelong testosterone exposure on health and disease using Mendelian randomization. Elife. 2020;9: e58914.
Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, Beaumont RN, Wittemans L, Martin S, Busch AS, Erzurumluoglu AM, Hollis B, O’Mara TA, The Endometrial Cancer Association Consortium, McCarthy MI, Langenberg C, Easton DF, Wareham NJ, Burgess S, Murray A, Ong KK, Frayling TM, Perry JRB. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26:252–258.
Yeasage JA, Davidson J, Widrow L, Berger PA. Plasma testosterone levels, depression, sexuality, and age. Biol Psychiatry. 1985;20:222–5.
Dwyer JB, Aftab A, Radhakrishnan R, Widge A, Rodriquez CI, Carpenter LL, Nemeroff CB, McDonald WM, Kalin NH. APA Council of Research Task Force on Novel Biomarkers and Treatments. Am J Psychiatry. 2020;177:686–705.
Yeap BB. Hormonal changes and their impact on cognition and mental health of ageing men. Maturitas. 2014;79:227–35.
Barrett-Connor E, Von Muhlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1999;84:573–7.
Shores MM, Sloan KL, Matsumoto AM, Moceri VM, Felker B, Kivlahan DR. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry. 2004;61:162–7.
McIntyre RS, Mancini D, Eisfeld BS, Soczynska JK, Grupp L, Konarski JZ, Kennedy SH. Calculated bioavailable testosterone levels and depression in middle-aged men. Psychoneuroendocrinology. 2006;31:1029–35.
Almeida OP, Yeap BB, Hankey GJ, Jamrozik K, Flicher L. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch Gen Psychiatry. 2008;65:283–9.
Joshi D, van Schoor NM, de Ronde W, Schaap LA, Comijs HC, Beekman ATF, Lips P. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin Endocrinol. 2010;72:232–40.
Ford AH, Yeap BB, Flicker L, Hankey GJ, Chubb SAP, Handelsmann DJ, Golledge J, Almeida OP. Prospective longitudinal study of testosterone and incident depression in older men: the Health in Men Study. Psychoneuroendocrinology. 2016;64:57–63.
Wainberg M, Kloiber S, Diniz B, McIntyre RS, Felsky D, Tripathy SJ. Clinical laboratory tests and five-year incidence of major depressive disorder: a prospective cohort study of 433,890 participants in the UK Biobank. Transl Psychiatry. 2021;11:380.
Aydogan U, Aydogdu A, Akbulut H, Sonmez A, Yuksel S, Basaran Y, Uzun O, Bolu E, Saglam K. Increased frequency of anxiety, depression, quality of life and sexual life in young hypogonadotropic hypogonadal males and impacts of testosterone replacement therapy on these conditions. Endocr J. 2012;59:1099–105.
Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract. 2009;15:289–305.
Fischer S, Ehlert U, Castro RA. Hormones of the hypothalamic-pituitary-gonadal (HPG) axis in male depressive disorders – a systematic review and meta-analysis. Front Neuroendocrinol. 2019;55: 100792.
O’Hara L, Curley M, Ferreira MT, Cruickshanks L, Milne L, Smith LB. Pituitary androgen receptor signalling regulates prolactin but not gonadotrophins in the male mouse. PLoS One. 2015;e10:e0121657.
Kaprara A, Huhtaniemi IT. The hypothalamus-pituitary-gonad axis: tales of mice and men. Metabolism. 2018;86:3–17.
Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–43.
Mhaouty-Kodja S. Role of the androgen receptor in the central nervous system. Mol Cell Endocrinol. 2018;465:103–12.
Comninos AN, Dhillo WS. Emerging roles of kisspeptin in sexual and emotional brain processing. Neuroendocrinology. 2018;106:195–202.
Schweiger U, Deuschle M, Weber B, Korner A, Lammers C-H, Schmider J, Gotthardt U, Heuser I. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med. 1999;61:292–6.
Young EA, Korszun A. The hypothalamic-pituitary-gonadal axis in mood disorders. Endcrinol Metab Clin North Am. 2002;31:63–78.
Amsterdam JD, Winokur A, Caroff S, Snyder P. Gonadotropin release after administration of GnRH in depressed patients and healthy volunteers. J Affect Disord. 1981;3:367–80.
Labrie F. Combined blockade of testicular and locally made androgens in prostate cancer: a highly significant medical progress based upon intracrinology. J Steroid Biochem Mol Biol. 2015;145:144–56.
Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–44.
Melloni C, Nelson A. Effect of androgen deprivation therapy on metabolic complications and cardiovascular risk. J Cardiovasc Transl Res. 2020;13:451–62.
Russell N, Grossmann M. Management of bone and metabolic effects of androgen deprivation therapy. Urol Oncol. 2021;39:704–12.
Narayan V, Harrison M, Cheng H, Kenfield S, Aggarwal R, Kwon D, McKay R, Hauger R, Hart N, Conzen S, Borno H, Jim H, Dicker A, Dorff T, Moslehi J, Mucci L, Parsons JK, Saad F, Soule H, Morgans A, Ryan CJ. Improving research for prostate cancer survivorship: a statement from the Survivorship Research in Prostate Cancer (SuRECaP) working group. Urol Oncol. 2020;38:83–93.
Nead KT. Androgens and depression: a review and update. Curr Opin Endocrinol Diabetes Obes. 2019;28:175–9.
Siebert AL, Lapping-Carr L, Morgans AK. Neuropsychiatric impact of androgen deprivation therapy in patients with prostate cancer: current evidence and recommendations for the clinician. Eur Urol Focus. 2020;6:1170–9.
Friberg AS, Dalton SO, Larsen SB, Andersen EW, Kroyer A, Helgstrand JT, Roder MA, Johansen C, Brasso K. Risk of depression after radical prostatectomy – a nationwide registry-based study. Eur Urol Oncol. 2021;4:601–8.
Thomas HR, Chen M-H, D’Amico AV, Bennett CL, Kattan MW, Sartor O, Stein K, Nguyen PL. Association between androgen deprivation therapy and patient-reported depression in men with recurrent prostate cancer. Clin Genitourin Cancer. 2018;16:313–7.
Stone P, Hardy J, Huddart R, A’Hern R, Richards M. Fatigue in patients with prostate cancer receiving hormone therapy. Eur J Cancer. 2000;36:1134–41.
Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology. 2004;29:1071–81.
Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology. 2009;18:237–47.
Nead KT, Sinha S, Yang DD, Nguyen PL. Association of androgen deprivation therapy and depression in the treatment of prostate cancer: a systematic review and meta-analysis. Urol Oncol. 2017;35:664.e1-664.e9.
Lee M, Jim HS, Fishman M, Zachariah B, Heysek R, Biagioli M, Jacobsen PB. Depressive symptomatology in men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Psychooncology. 2015;24:472–7.
Pirl WF, Siegel GI, Goode MJ, Smith MR. Depression in men receiving androgen deprivation therapy for prostate cancer: a pilot study. Psychooncology. 2002;11:518–23.
Saini A, Berrui A, Cracco C, Squazzotti E, Porpiglia F, Russo L, Bertaglia V, Picci RL, Negro M, Tosco A, Campagna S, Scarpa RM, Dogliotti L, Furlan PM, Ostacoli L. Psychological distress in men with prostate cancer receiving adjuvant androgen-deprivation therapy. Urol Oncol. 2013;31:352–8.
Shin D, Shim SR, Kim CH. Changes in Beck depression inventory scores in prostate cancer patients undergoing androgen deprivation therapy or prostatectomy. PLoS ONE. 2020;15: e0234264.
Chung S-D, Kao L-T, Lin H-C, Xirasagar S, Huang C-C, Lee H-C. Patients receiving androgen deprivation therapy for prostate cancer have an increased risk of depressive disorder. PLoS ONE. 2017;12: e0173266.
Pirl WF, Greer JA, Goode M, Smith MR. Prospective study of depression and fatigue in men with advanced prostate cancer receiving hormone therapy. Psychooncology. 2008;17:148–53.
Timilshina N, Breunis H, Alibhai S. Impact of androgen deprivation therapy on depressive symptoms in men with nonmetastatic prostate cancer. Cancer. 2012;118:1940–5.
Deka R, Rose BS, Bryant AK, Sarkar RR, Nalawade V, McKay R, Murphy JD, Simpson DR. Androgen deprivation therapy and depression in men with prostate cancer treated with definitive radiation therapy. Cancer. 2019;125:1070–80.
Dinh KT, Reznor G, Muralidhar V, Mahal BA, Nezolosky MD, Choueiri TK, Hoffman KE, Hu JC, Sweeney CJ, Trinh Q-D, Nguyen PL. Association of androgen deprivation therapy with depression in localized prostate cancer. J Clin Oncol. 2016;34:1905–12.
Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med. 2006;166:465–71.
Tully KH, Nguyen D-D, Herzog P, Jin G, Noldus J, Nguyen PL, Kibel AS, Sun M, McGregor B, Basaria S, Trinh Q-D. Risk of dementia and depression in young and middle-aged men presenting with nonmetastatic prostate cancer treated with androgen deprivation therapy. Eur Urol Oncol. 2021;4:66–72.
Nowakowska MK, Lei X, Wehner MR, Corn PG, Giordano SH, Nead KT. Association of second-generation antiandrogens with depression among patients with prostate cancer. JAMA Netw Open. 2021;4: e2140803.
Matsumoto AM. Testosterone replacement in men with age-related low testosterone: what did we learn from the Testosterone Trials? Curr Opin Endocr Metab Res. 2019;6:34–41.
Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Resnick SM, Budoff M, Mohler ER 3rd, Wenger NK, Cohen HJ, Schrier S, Keaveny TM, Kopoperdahl D, Lee D, Cifelli D, Ellenberg SS. Lessons from the Testosterone Trials. Endocr Rev. 2018;39:369–86.
Yeap BB, Page ST, Grossmann M. Testosterone treatment in older men: clinical implications and unresolved questions from the Testosterone Trials. Lancet Diabetes Endocrinol. 2018;6:659–72.
Budoff MJ, Ellenberg SS, Lewis CE, Mohler ER 3rd, Wenger N, Bhasin S, Barrett-Connor E, Swerdloff RS, Stephens-Shields A, Cauley JA, Crandall JP, Cunningham GR, Ensrud KE, Gill TM, Matsumoto AM, Molitch ME, Nakanishi R, Nezarat N, Matsumoto S, Hou X, Basaria S, Diem SJ, Wang C, Cifelli D, Snyder PJ. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317:708–16.
Roy CN, Snyder PJ, Stephens-Shields AJ, Stephens-Shields AJ, Artz AS, Bhasin S, Cohen HJ, Farrar JT, Gill TM, Zeldow B, Cella D, Barrett-Connor E, Cauley JA, Crandall JP, Cunningham GR, Ensrud KE, Lewis CE, Matsumoto AM, Molitch ME, Pahor M, Swerdloff RS, Cifelli D, Hou X, Resnick SM, Walston JD, Anton S, Basaria S, Diem SJ, Wang C, Schrier SL, Ellenberg SS. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med. 2017;177:480–90.
Amanatkar HR, Chiball JT. Impact of exogenous testosterone on mood: a systematic review and meta-analysis of randomized placebo-controlled trials. Ann Clin Psychiatry. 2014;26:19–32.
Elliott J, Kelly SE, Millar AC, Peterson J, Chen L, Johnston A, Koth A, Skidmore B, Bai Z, Mamdani M, Wells GA. Testosterone therapy in hypogonadal men: a systematic review and network meta-analysis. BMJ Open. 2017;7: e015284.
dos Santos MR, Bhasin S. Benefits and risks of testosterone treatment in men with age-related decline in testosterone. Annu Rev Med. 2021;72:75–91.
Vartolomei MD, Kimura S, Vartomomei L, Shariat SF. Systematic review of the impact of testosterone replacement therapy on depression in patients with late-onset testosterone deficiency. Eur Urol Focus. 2020;6:170–7.
Walther A, Breidenstein J, Miller R. Association of testosterone treatment with alleviation of depressive symptoms in men: a systematic review and meta-analysis. JAMA Psychiat. 2019;76:31–40.
Dean JD, Carnegie C, Rodzvilla J, Smith T. Long-term effects of Testim 1% gel in hypogonadal men. Rev Urol. 2005;7:87–94.
McNichols TA, Dean JD, Mulder H, Carnegie C, Jones NA. A novel testosterone gel formulation normalizes androgen levels in hypogonadal men with improvement in body composition and sexual function. BJU Int. 2003;91:69–74.
Giltay EJ, Tishova YA, Mskhalaya GJ, Gooren LJG, Saad F, Kalinchenko SY. Effects of testosterone supplementation on depressive symptoms and sexual dysfunction in hypogonadal men with metabolic syndrome. J Sex Med. 2010;7:2572–82.
Pope HG, Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry. 2003;160:105–11.
Seidman SN, Orr G, Raviv G, Levi R, Roose SP, Kravitz E, Amiaz R, Weiser M. Effects of testosterone replacement in middle-aged men with dysthymia: a randomized, placebo-controlled clinical trial. J Clin Psychopharmacol. 2009;29:216–21.
Shores MM, Kivlahan DR, Sadak TI, Li EJ, Matsumoto AM. A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression). J Clin Psychiatry. 2009;70:1009–16.
Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Cifelli D, Dougar D, Fluharty L, Resnick SM, Storer TW, Anton S, Basaria S, Diem SJ, Hou X, Mohler ER III, Parsons JK, Wenger NK, Zeldow B, Landis JR, Ellenberg SS. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–24.
Bhasin S, Seidman S. Testosterone treatment of depressive disorders in men. JAMA Psychiat. 2019;76:9–10.
Bennett NC, Gardner RA, Hooper JD, Johnson DW, Gobe GC. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol. 2010;42:813–27.
Davey RA, Grossmann M. Androgen receptor structure, function and biology: from bench to bedside. Clin Biochem Rev. 2016;37:3–15.
Matsumoto T, Sakari M, Okada M, Yokoyama A, Takahashi S, Kouzmenko A, Kato S. The androgen receptor in health and disease. Annu Rev Physiol. 2013;75:201–24.
Fernandez-Guasti A, Kruijver FPM, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol. 2000;425:422–35.
Roselli CE, Klosterman S, Resko JA. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J Comp Neurol. 2001;439:208–23.
Wang S-S, Kamphuis W, Huitinga I, Zhou J-N, Swaab DF. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry. 2008;13:786–99.
Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142–8.
Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. 2008;29:169–81.
Heberden C. Sex steroids and neurogenesis. Biochem Pharmacol. 2017;141:56–62.
Jaaskelainen J. Molecular biology of androgen insensitivity. Mol Cell Endocrinol. 2012;352:4–12.
Mueller SC, Grissom EM, Dohanich GP. Assessing gonadal hormone contributions to affective psychopathologies across humans and animal models. Psychoneuroendocrinology. 2014;46:114–28.
Ryan CP, Crespi BJ. Androgen receptor polyglutamine repeat number: models of selection and disease susceptibility. Evol Appl. 2012;6:180–96.
Yeap BB, Wilce JA, Leedman PJ. The androgen receptor mRNA BioEassay. 2004;26:672–82.
Saunderson RB, Yu B, Trent RJA, Pamphlett R. A comparison of the lengths of androgen receptor triplet repeats in brain and blood in motor neuron diseases. J Neurol Sci. 2008;267:125–8.
Crabbe P, Bogaert V, De Bacquer D, Goemaere S, Zmierczak H, Kaufman JM. Part of the interindividual variation in serum testosterone levels in healthy men reflects differences in androgen sensitivity and feedback set point: contribution of the androgen receptor polyglutamine tract polymorphism. J Clin Endocrinol Metab. 2007;92:3604–10.
Colangelo LA, Sharp L, Kopp P, Schlotens D, Chiu BC-H, Liu K, Gapstur SM. Total testosterone, androgen receptor polymorphism, and depressive symptoms in young black and white men: the CARDIA Male Hormone Study. Psychoneuroendocrinology. 2007;32:951–958.
Sankar JS, Hampson E. Testosterone levels and androgen receptor gene polymorphism predict specific symptoms of depression in young men. Gend Med. 2012;9:232–43.
Seidman SN, Araujo AB, Roose SP, McKinlay JB. Testosterone level, androgen receptor polymorphism, and depressive symptoms in middle-aged men. Biol Psychiatry. 2001;50:371–6.
Hirtz R, Libuda L, Hinney A, Focker M, Buhlmeier J, Holterhus P-M, Kulle A, Kiewert C, Hebebrand J, Grasemann C. Size matters: the CAG repeat length of the androgen receptor gene, testosterone, and male adolescent depression study. Front Psychiatry. 2021;12: 732759.
Schneider G, Nienhaus K, Gromoll J, Heuft G, Nieschlag E, Zitzmann M. Depressive symptoms in men aged 50 years and older and their relationship to genetic androgen receptor polymorphism and sex hormone levels in three different samples. Am J Geriatr Psychiatry. 2011;19:274–83.
Harkonen K, Huhtaniemi I, Markinen J, Hubler D, Irjala K, Koskenvuo M, Oettel M, Raitakari O, Saad F, Pollanen P. The polymorphic androgen receptor gene CAG repeat, pituitary-testicular function and andropausal symptoms in ageing men. Int J Androl. 2003;26:187–94.
Azad N, Pitale S, Barnes WE, Friedman N. Testosterone treatment enhances regional brain perfusion in hypogonadal men. J Clin Endocrinol Metab. 2003;88:3064–8.
Hofer P, Lanzenberger R, Kasper S. Testosterone in the brain: neuroimaging findings and the potential role for neuropsychopharmacology. Eur Neuropsychopharmacol. 2013;23:79–88.
Moffat SD, Resnick SM. Long-term measures of free testosterone predict regional cerebral blood flow patterns in elderly men. Neurobiol Aging. 2007;28:914–20.
Hajszan T, MacLusky NJ, Leranth C. Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Horm Behav. 2008;53:638–546.
Harley CW, Malsbury CW, Squires A, Brown RA. Testosterone decreases CA1 plasticity in vivo in gonadectomized male rats. Hippocampus. 2000;10:693–7.
Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spinek synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–92.
Galea LA, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–32.
Hung Y-Y, Huang Y-L, Chang C, Kang H-Y. Deficiency in androgen receptor aggravates the depressive-like behaviors in chronic mild stress model of depression. Cells. 2019;8:1021.
Carrier N, Kabbaj M. Extracellular signal-regulated kinase 2 signaling in the hippocampal dentate gyrus mediates the antidepressant effects of testosterone. Biol Psychiatry. 2012;71:642–51.
Nautiyal KM, Hen R. Serotonin receptors in depression: from A to B. F1000Res. 2017;6:123.
McQueen JK, Wilson H, Sumner BEH, Fink G. Serotonin transporter (SERT) mRNA and binding site densities in male rat brain affected by sex steroids. Brain Res Mol Brain Res. 1999;63:241–7.
Robichaud M, Debonnel G. Oestrogen and testosterone modulate the firing activity of dorsal raphe nucleus serotonergic neurons in both male and female rats. J Neuroendocrinol. 2005;17:179–85.
Perfalk E, da Cunha-Bang Sofi, Holst KK, Keller S, Svarer C, Knudsen GM, Frokjaer VG. Testosterone levels in healthy men correlate negatively with serotonin 4 receptor binding. Psychoneurendocrinology. 2017;81:22–28.
Kranz GS, Spies M, Vraka C, Kaufman U, Klebermass E-M, Handschuh PA, Ozenil M, Murgas M, Pichler V, Rischka L, Nics L, Konadu ME, Ibeschitz H, Traub-Weidinger T, Wadsak W, Hahn A, Hacker M, Lanzenberger R. High-dose testosterone treatment reduces monoamine oxidase A levels in the human brain: a preliminary report. Psychoneuroendocrinology. 2021;133: 105381.
Meyers B, D’Agostino A, Walker J, Kritzer MF. Gonadectomy and hormone replacement exert region- and enzyme isoform-specific effects on monoamine oxidase and catechol-o-methyl transferase activity in prefrontal and neostriatum of adult male rats. Neuroscience. 2010;165:850–62.
Mulvey B, Dougherty JD. Transcriptional-regulatory convergence across functional MDD risk variants identified by massively parallel reporter assays. Transl Psychiatry. 2021;11:403.
Ring HZ, Lessov CN, Reed T, Marcus R, Holloway L, Swan GE, Carmelli D. Heritability of plasma sex hormones and hormone binding globulin in adult male twins. J Clin Endocrinol Metab. 2005;90:3653–8.
Kuijper EAM, Lambalk CB, Boomsma DI, van der Sluis S, Blankenstein MA, de Geus EJC, Posthuma D. Heritability of reproductive hormones in adult male twins. Hum Reprod. 2007;22:2153–9.
Pagadala MS, Jasuja GK, Palnati P, Lynch J, Anglin T, Chang N, Deka R, Lee KM, Agiri FY, Seibert TM, Rose BS, Carter H, Panizon MS, Hauger RL. Discovery of novel trans-ancestry and ancestry-specific gene loci for total testosterone in a multi-ancestral analysis of men in the Million Veteran Program. medRxiv. 2022;16.21265846
Mularoni V, Esposito V, Di Persio S, Vicini E, Spadetta G, Berloco P, Fanelli F, Mezzullo M, Pagotto U, Pelusi C, Nielsen JE, Rajpert-De Meyts E, Jorgensen N, Jorgenson A, Biotani C. Age-related changes in human Leydig cell status. Hum Reprod. 2020;35:2663–76.
Acknowledgements
We acknowledge funding support from Million Veteran Program MVP022 (CX001727) grant from Office of Research and Development (ORD) of the US Department of Veterans Affairs and from NIA RO1 AG050595 grant, and NIA RO1 AG05064 grant from the National Institute of Aging of the National Institutes of Health for Richard L. Hauger (RLH) and Matthew S. Panizzon (MSP). In addition, RLH was also funded by the Center of Excellence for Stress and Mental Health (CESAMH) from ORD of the US Department of Veterans Affairs and Meghana S. Pagadala (MSP) was funded by Million Veteran Program MVP022 (CX001727). This publication does not represent the views of the Department of Veterans Affairs or the United States Government.
Funding
The authors have no financial, non-financial, or other relevant conflicts of interest to disclose that are directly or indirectly related to the work submitted for publication.
Author information
Authors and Affiliations
Contributions
Richard L. Hauger (RLH) conceptualized the review, completed a comprehensive search of relevant literature, wrote the initial draft, and revised and finalized the manuscript. Ursula G. Saelzler (UGS) contributed to the literature review, wrote several sections of the manuscript, and edited the final version. Meghana S. Pagadala (MSP) provided important information about gene variants regulating testosterone that were identified in the GWAS of morning testosterone levels she completed. Meghana S. Pagadala (MSP) and Matthew S. Panizzon (MSP) contributed important ideas and interpretation of relevant literature.
Corresponding author
Ethics declarations
Ethical approval and informed consent
This review does not require any ethical approval or informed consent.
Conflict of interest
The corresponding author Richard L. Hauger (RLH) has no relevant conflicts of interest to disclose interests that are directly or indirectly related to the work submitted for publication. Ursula G. Saelzler (UGS), Meghana S. Pagadala (MSP), and Matthew S. Panizzon (MSP) have no relevant conflicts to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hauger, R.L., Saelzler, U.G., Pagadala, M.S. et al. The role of testosterone, the androgen receptor, and hypothalamic-pituitary–gonadal axis in depression in ageing Men. Rev Endocr Metab Disord 23, 1259–1273 (2022). https://doi.org/10.1007/s11154-022-09767-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-022-09767-0