Abstract
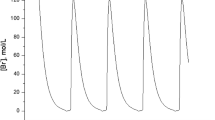
A kinetic method for the determination of l-tyrosine (Tyr) by analyte pulse perturbation, caused by different amounts of Tyr, in the Bray–Liebhafsky (BL) oscillatory reaction realized in a continuous-flow stirred tank reactor, is proposed. For such purposes, the BL oscillating reaction was kept in a stable non-equilibrium stationary state close to the bifurcation point and was used as the matrix reaction system. Under optimum reaction conditions, the linear relationship between the kinetic parameters, ∆τ2 (the period between first two oscillations that appear after applied perturbation) as well as τend (the time elapsed between the perturbation of the BL reaction by Tyr and the termination of the oscillatory phase) and the Tyr concentrations is obtained over the ranges 1.1 × 10−6 − 9.2 × 10−6 mol L−1 and 1.1 × 10−6 − 1.4 × 10−5 mol L−1, with the limit of detections of 6.6 × 10−7 mol L−1 and 6.4 × 10−7 mol L−1. The described method that relies on a simple instrumental set-up, has been successfully applied to the determination of Tyr in a dietary supplement. Some aspects of the possible mechanism of Tyr action on the BL oscillator are discussed.




Similar content being viewed by others
References
Gray P, Scott SK (1990) Chemical oscillations and instabilities: nonlinear chemical kinetics. Clarendon Press, Oxford
Epstein IR, Rastogi RP (2008) Introduction to non-equilibrium physical chemistry-towards complexity and non-linear science. Elsevier, Amsterdam
Kolar-Anić Lj, Anić S, Čupić Ž, Ivanović-Šašić A, Pejić N, Blagojević S, Vukojević V (2017) In: Wang Z (ed) Wille U, Juaristi E (ass. eds), Chapter 23 Oscillating reactions in encyclopedia of physical organic chemistry, 6 vol Set, vol 2, Part 2 Organic reactions and mechanisms. Wiley, New York, pp 1127–1222
Field RJ, Burger M (eds) (1985) Oscillation and traveling waves in chemical systems. Wiley, New York
Bray WC (1921) Periodic reaction in homogenous solution and its relation to catalysis. J Am Chem Soc 43:1262–1267
Bray WC, Liebhafsky HA (1931) Reaction involving hydrogen peroxide, iodine, and iodate ion I. Introduction. J Am Chem Soc 53:38–44
Jiménez-Prieto R, Silva M, Pérez-Bendito D (1998) Approaching the use of oscillating reactions for analytical monitoring. Analyst 123:1R-8R
Gao J (2005) Application of oscillating chemical reaction to analytical chemistry: recent development. Pak J Biol Sci 8:512–519
Pejić N (2009) Analytical applications of pulse perturbation method of Bray–Liebhafsky oscillatory reaction realized in the open reactor. Hem Ind 63:455–466 ((in Serbian))
Ren J, Zhang X, Gao J, Yang W (2013) The application of oscillating chemical reactions to analytical determinations. Cent Eur J Chem 11(7):1023–1031
Makismović J, Čupić Ž, Manojlović N, Đerić A, Anić S, Lj K-A (2020) Bray-Liebhafsky oscillatory reaction as the matrix system for the kinetic determination of microquantities of alizarin and purpurin. React Kinet Mech Catal 130:655–668
Anić S, Maksimović J, Lončarevi D, Pejić N, Čupić Ž (2009) Activity of polymer supported cobalt catalyst in the Bray–Liebhafsky oscillator. Russ J Phys Chem A 83(9):1468–1472
Maksimović JP, Čupić ŽD, Lončarević D, Pejić N, Vasiljević-Radović D, Anić S (2011) Kinetics of the Bray–Liebhafsky oscillatory reaction perturbed by polymer supported cobalt catalyst. Sci Sinter 43:55–62
Vukojević V, Anić S, Lj K-A (2000) Investigation of dynamic behavior of the Bray–Liebhafsky reaction in the CSTR. Determination of bifurcation points. J Phys Chem A 104:10731–10739
Jiménez-Prieto R, Silva M, Pérez-Bendito D (1995) Analyte pulse perturbation technique: a tool for analytical determinations in far-from-equilibrium dynamic systems. Anal Chem 67:729–734
Vukojević V, Pejić N, Stanisavljev D, Anić S, Lj K-A (1999) Determination of Cl−, Br−, I−, Mn2+, malonic acid and quercetin by perturbation of a non-equilibrium stationary state in the Bray–Liebhafsky reaction. Analyst 124:147–152
Pejić N, Anić S, Kuntić V, Vukojević V, Lj K-A (2003) Kinetic determination of microquantities of rutin by perturbation of the Bray–Liebhafsky oscillatory reaction in an open system. Microchim Acta 143:261–267
Pejić N, Blagojević S, Anić S, Vukojević V, Lj K-A (2005) Microquantitative determination of hesperidin by pulse perturbation of the oscillatory reaction system. Anal Bioanal Chem 381:775–780
Pejić N, Lj K-A, Anić S, Stanisavljev D (2006) Determination of paracetamol in pure and pharmaceutical dosage forms by pulse perturbation technique. J Pharm Biomed Analy 41:610–615
Pejić N, Blagojević S, Anić S, Vukojević V, Mijatović M, Ćirić J, Marković Z, Marković S, Lj K-A (2007) Kinetic determination of morphine by means of Bray–Liebhafsky oscillatory reaction system using analyte pulse perturbation technique. Anal Chim Acta 582:367–374
Pejić N, Blagojević S, Vukelić J, Lj K-A, Anić S (2007) Analyte pulse perturbation tecnique for the determination of 6-monoacetylmorphine in seized street drug sample. Bull Chem Soc Jpn 80(10):1942–1948
Pejić N, Blagojević S, Anić S, Lj K-A (2007) Determination of ascorbic acid in pharmaceutical dosage forms and urine by means of an oscillatory reaction system using the pulse perturbation technique. Anal Bioanal Chem 389:2009–2017
Maksimović JP, LjZ K-A, Anić SR, Ribič DD, Pejić ND (2011) Quantitative determination of some water-soluble B vitamins by kinetic analytical method based on the perturbation of an oscillatory reaction. J Braz Chem Soc 22:38–48
Pejić ND, Maksimović JP, Blagojević SM, Anić SR, Čupić ŽD, LjZ K-A (2012) Kinetic analytical method for determination of uric acid in human urine using analyte pulse perturbation technique. J Braz Chem Soc 23(8):1450–1459
Pejić ND, Sarap NB, Maksimović JP, Anić SR, LjZ K-A (2013) Pulse perturbation technique for determination of piroxicam in pharmaceuticals using an oscillatory reaction system. Cent Eur J Chem 11:180–188
Ma Y-J, Dong W-B, Fan C, Wang E-D (2017) Identification of cow milk in goat milk by nonlinear chemical fingerprint technique. J Food Drug Anal 25:751–758
Pagnacco M, Maksimović J, Mudrinić T, Banković P, Nedić-Vasiljević B, Milutinović-Nikolić A (2020) Oscillatory Briggs–Rauscher reaction as “fingerprint” for bentonite identification: the fine-tuning of oscillatory dynamics with addition of clay. ChemistrySelect 5:8137–8141
Cervellati R (2021) William Crowell Bray and the discovery of the first periodic homogeneous reaction in 1921. React Kinet Mech Catal. https://doi.org/10.1007/s11144-021-02019-3
Pejić N, Maksimović J, Ribič D, Lj K-A (2009) Dynamic states of the Bray–Liebhafsky reaction when sulfuric acid is the control parameter. Russ J Phys Chem A 83:1666–1671
Pejić N, Vujković M, Maksimović J, Ivanović A, Anić S, Ćupić Ž, Lj K-A (2011) Dynamic behavior of the Bray–Liebhafsky oscillatory reaction controlled by sulfuric acid and temperature. Russ J Phys Chem A 85:2310–2316
Pejić N, Lj K-A, Maksimović J, Janković M, Vukojević V, Anić S (2016) Dynamic transitions in the Bray–Liebhafsky oscillating reaction. Effect of hydrogen peroxide and temperature on bifurcation. Reac Kinet Mech Cat 118:15–26
Bubanja IN, Maćešić S, Ivanović-Šašić A, Čupić Ž, Anić S, Lj K-A (2016) Intermittent chaos in the Bray–Liebhafsky oscillator. Temperature dependence Phys Chem Chem Phys 18:9770–9778
Ganaie NB, Peerzada GM (2017) Dynamical regime of resorcinol based Belousov–Zhabotinsky chemical oscillator in the presence or absence of some hydrophobic antioxidants in aqueous–organic mixed media. RSC Adv 7:13019–13031
Li H, Xu Y, Wang M (2002) Several types of oscillations in Belousov–Zhabotinskii reactions with amino acids as organic substrates. Inter J Chem Kinet 34:405–410
Li H, Zhu J, Dai Y, Wang Q (2003) Iodide-induced oscillations in Br O3−–Tyrosine–H2SO4 system. Chem Lett 32:158–159
Li Z, Yuan C, Nie F (2005) Kinetic parameters of oscillating reaction of amino acid–Br O3−–Mn2+–H2SO4–acetone system. Chin Sci Bull 50:15–20
Nussey S, Whitehead S (2001) Endocrinology, an intergrated approach. Oxford BIOS Scientific Publishers, Oxford
Pal GK (2007) Textbook of medical physiology, endocrine physiology. Ahuja Publishing House, India
Ahad F, Ganie SA (2010) Iodine, iodine metabolism and iodine deficiency disorder revisited. Indian J Endocrinol Metab 14:13–17
Hardy PM (1985) The protein amino acids. In: Barrett GC (ed) Chemistry and biochemistry of the amino acids. Springer, pp 6–24
Currie PJ, Chang N, Luo S, Anderson GH (1995) Microdialysis as a tool to measure dietary and regional effects on the complete profile of extracellular amino acids in the hypothalamus of rats. Life Sci 57:1911–1923
Silvàn JM, van de Lagemaat OA, del Castilo MD (2006) Analysis and biological properties of amino acid derivates formed by Maillard reaction in foods. J Pharm Biomed Anal 41:1543–1551
Murtada K, Salghi R, Ríos A, Zougagh M (2020) A sensitive electrochemical sensor based on aluminium doped copper selenide nanoparticles-modified screen printed carbon electrode for determination of L-tyrosine in pharmaceutical samples. J Electroanal Chem 874:114466
Elshiekh B, Basheir A, Elbashir AA (2015) Spectrophotometric methods for the determination of L-tyrosine in pharmaceutical formulations. ChemExpress 8:95–101
Wei W, Wang H-J, Jiang C-Q, Shi J-M (2007) Spectrofluorimetric determination of trace amounts of tyrosine with 1,5-Bis(4,6-dicholro-1,3,5-triazinylamino) naphthalene. Chin J Anal Chem 35:1772–1775
Neurauter G, Scholl-Bürgi S, Haara A, Geisler S, Mayersbach P, Schennach H, Fuchs D (2013) Simultaneous measurement of phenylalanine and tyrosine by high performance liquid chromatography (HPLC) with fluorescence detection. Clin Biochem 46:1848–1851
Felitsyn NM, Henderson GN, James MO, Stacpoole PW (2004) Liquid chromatography-tandem mass spectrometry method for the simultaneous determination of y-ALA, tyrosine and creatinine in biological fuids. Clin Chim Acta 350:219–230
Chen G, Ye JN, Cheng JS (2000) Determination of monoamine transmitters and tyrosine in biological samples by capillary electrophoresis with electrochemical detection. Chromatographia 52:137–141
Varmira K, Mohammadi G, Mahmoudi M, Khodarahmi R, Rashidi K, Hedayati M, Goicoechea HC, Jalalvand AR (2018) Fabrication of a novel enzymatic electrochemical biosensor for determination of tyrosine in some food samples. Talanta 183:1–10
Zou H-Y, Lu X-Y, Kong F-Y, Wang Z-X, Li H-Y, Fang H-L, Wang W (2020) A voltammetric sensor based on reduced graphene oxide-hemin-Ag nanocomposites for sensitive determination of tyrosine. RSC Adv 10:28026–28031
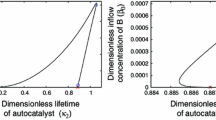
Stanković B, Čupić Ž, Maćešić S, Pejić N, Lj K-A (2016) Complex bifurcations in the oscillatory reaction model. Chaos Solit Fractals 87:84–91
ICH Guideline Q2B (1997)
Desimoni E, Brunetti B (2015) About estimating the limit of detection by the signal to noise approach. Pharm Anal Acta 6:4
Čudina I, Lj J (1987) The effect of oxygen on the radiolysis of tyrosine in aqueous solutions. Radiat Res 109:206–215
Neyra Recky JR, Serrano MP, Dántola ML, Lorente C, (2021) Oxidation of tyrosine: antioxidant mechanism of L-DOPA disclosed. Free Radic Biol Med 165:360–367
Houée-Lévin C, Bobrowski K, Horakova L, Karademir B, Schöneich C, Davies MJ, Spickett CM (2015) Exploring oxidative modifi cations of tyrosine: an update on mechanisms of formation, advances in analysis and biological consequences. Free Radic Res 49:347–373
Dunford B, Ralston I (1983) On the mechanism of iodination of tyrosine. Biochem Biophys Res Commun 116:639–643
Aghaie M, Miryaie M, Zare K, Manajjemi M, Aghaie H (2008) Kinetic and mechanism studies of the reaction between L-tyrosine and iodine on the basis of UV-VIS spectrophotometric method. Asian J Biochem 3:290–298
Kumar K, Woolum K (2021) A novel reagent for radioiodine labeling of new chemical entities (NCEs) and biomolecules. Molecules 26:4344
Sun W, Dunford BH (1993) Kinetics and mechanism of the peroxidase-catalyzed iodination of tyrosine. Biochem 32:1324–1331
Acknowledgements
The authors are grateful Dr. Ana Stanojević and Dr. Maja Pagnacco on initial organization of these investigations. This work was partially supported by the Ministry of Education, Science and Technological Development of Republic of Serbia (Grant Numbers NES 7743504, OI 172015 and III 45001, and Contract Numbers 451-03-9/2021-14/200146, 451-03-9/2021-14/200026 and 451-03-9/2021-14/200161).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Negrojević, L., Lončar, A., Maksimović, J. et al. Bray–Liebhafsky oscillatory reaction in a continuous-flow stirred tank reactor as the matrix system for determination of tyrosine. Reac Kinet Mech Cat 135, 1147–1162 (2022). https://doi.org/10.1007/s11144-021-02130-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02130-5