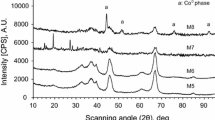
Abstract
In this research work, a simple, one-step and environmentally friendly method is reported for synthesizing metallic Co nanoparticles (NPs) and its application in Fischer–Tropsch Synthesis. In this attractive method, metallic CoNPs decorated on the alumina surface are prepared by auto-reduction of cobalt (II) formate dihydrate at 380 °C for 4 h under high-pure nitrogen atmosphere. During the thermal decomposition process, hydrogen and carbon monoxide gases, which are reducing agents, are released. The decomposed samples were directly used in the reaction without typical hydrogen pretreatment. For comparison, the Co/γ-Al2O3 catalyst was also prepared by the traditional impregnation method, which includes the calcination and reduction steps. Different characterization techniques including TGA-DTA, XRD, FESEM, EDX mapping and BET were carried out to investigate the structural and textural properties of samples. CO conversion of the auto-reduced Co/γ-Al2O3 catalyst was almost twofold of the reduced catalyst by H2. The characterization data indicated that auto-reduction method leads to better dispersion and smaller CoNPs production than those prepared by H2 reduction pretreatment. Therefore, the active sites for the reaction increased, leading to an increase in CO conversion. The results suggested that the catalyst reduction under H2 stream is unnecessary.





Similar content being viewed by others
References
Li X, Hao X, Abudula A, Guan G (2016) Nanostructured catalysts for electrochemical water splitting: current state and prospects. J Mater Chem A 4(31):11973–12000. https://doi.org/10.1039/C6TA02334G
Wang H, Gao Q, Li H, Han B, Xia K, Zhou C (2019) One-pot synthesis of a novel hierarchical Co(II)-doped TiO2 nanostructure: toward highly active and durable catalyst of peroxymonosulfate activation for degradation of antibiotics and other organic pollutants. Chem Eng J 368:377–389. https://doi.org/10.1016/j.cej.2019.02.124
Roy P, Srivastava SK (2019) Nanomaterials for electrochemical energy storage devices. Wiley, Hoboken
Pei Y, Li Z, Li Y (2017) Highly active and selective Co-based Fischer-Tropsch catalysts derived from metal–organic frameworks. AIChE J 63(7):2935–2944. https://doi.org/10.1002/aic.15677
Cheng Q, Tian Y, Lyu S, Zhao N, Ma K, Ding T, Jiang Z, Wang L, Zhang J, Zheng L, Gao F, Dong L, Tsubaki N, Li X (2018) Confined small-sized cobalt catalysts stimulate carbon-chain growth reversely by modifying ASF law of Fischer-Tropsch synthesis. Nat Commun 9(1):3250. https://doi.org/10.1038/s41467-018-05755-8
Mandić M, Todić B, Živanić L, Nikačević N, Bukur DB (2017) Effects of catalyst activity, particle size and shape, and process conditions on catalyst effectiveness and methane selectivity for Fischer-Tropsch reaction: a modeling study. Ind Eng Chem Res 56(10):2733–2745. https://doi.org/10.1021/acs.iecr.7b00053
Rauch R, Kiennemann A, Sauciuc A (2013) Chapter 12 - Fischer-Tropsch synthesis to biofuels (BtL Process). In: Triantafyllidis KS, Lappas AA, Stöcker M (eds) The role of catalysis for the sustainable production of bio-fuels and bio-chemicals. Elsevier, Amsterdam, p 398
Li J, He Y, Tan L, Zhang P, Peng X, Oruganti A, Yang G, Abe H, Wang Y, Tsubaki N (2018) Integrated tuneable synthesis of liquid fuels via Fischer-Tropsch technology. Nat Catal 1(10):787–793. https://doi.org/10.1038/s41929-018-0144-z
Gnanamani MK, Hamdeh HH, Jacobs G, Qian D, Liu F, Hopps SD, Thomas GA, Shafer WD, Xiao Q, Hu Y, Davis BH (2016) Fischer-Tropsch synthesis: effect of Cu, Mn and Zn addition on activity and product selectivity of cobalt ferrite. RSC Adv 6(67):62356–62367. https://doi.org/10.1039/C6RA10150J
Rahmati M, Huang B, Schofield LM, Fletcher TH, Woodfield BF, Hecker WC, Bartholomew CH, Argyle MD (2018) Effects of Ag promotion and preparation method on cobalt Fischer-Tropsch catalysts supported on silica-modified alumina. J Catal 362:118–128. https://doi.org/10.1016/j.jcat.2018.03.027
Garcilaso V, Barrientos J, Bobadilla LF, Laguna OH, Boutonnet M, Centeno MA, Odriozola JA (2019) Promoting effect of CeO2, ZrO2 and Ce/Zr mixed oxides on Co/γ-Al2O3 catalyst for Fischer-Tropsch synthesis. Renew Energy 132:1141–1150. https://doi.org/10.1016/j.renene.2018.08.080
Bartholomew CH (2001) Mechanisms of catalyst deactivation. Appl Catal A Gen 212(1):17–60. https://doi.org/10.1016/S0926-860X(00)00843-7
Subramanian V, Cheng K, Lancelot C, Heyte S, Paul S, Moldovan S, Ersen O, Marinova M, Ordomsky VV, Khodakov AY (2016) Nanoreactors: an efficient tool to control the chain-length distribution in Fischer-Tropsch synthesis. ACS Catal 6(3):1785–1792. https://doi.org/10.1021/acscatal.5b01596
Prieto G, Martínez A, Concepción P, Moreno-Tost R (2009) Cobalt particle size effects in Fischer-Tropsch synthesis: structural and in situ spectroscopic characterisation on reverse micelle-synthesised Co/ITQ-2 model catalysts. J Catal 266(1):129–144. https://doi.org/10.1016/j.jcat.2009.06.001
Yang C, Zhao B, Gao R, Yao S, Zhai P, Li S, Yu J, Hou Y, Ma D (2017) Construction of synergistic Fe5C2/Co heterostructured nanoparticles as an enhanced low temperature Fischer-Tropsch synthesis catalyst. ACS Catal 7(9):5661–5667. https://doi.org/10.1021/acscatal.7b01142
Xie J, Paalanen PP, van Deelen TW, Weckhuysen BM, Louwerse MJ, de Jong KP (2019) Promoted cobalt metal catalysts suitable for the production of lower olefins from natural gas. Nat Commun 10(1):167. https://doi.org/10.1038/s41467-018-08019-7
Todic B, Bhatelia T, Froment GF, Ma W, Jacobs G, Davis BH, Bukur DB (2013) Kinetic model of fischer–tropsch synthesis in a slurry reactor on Co–Re/Al2O3 catalyst. Ind Eng Chem Res 52(2):669–679. https://doi.org/10.1021/ie3028312
Mosayebi A, Abedini R (2017) Detailed kinetic study of Fischer – Tropsch synthesis for gasoline production over CoNi/HZSM-5 nano-structure catalyst. Int J Hydrogen Energy 42(44):27013–27023. https://doi.org/10.1016/j.ijhydene.2017.09.060
den Otter JH, Nijveld SR, de Jong KP (2016) Synergistic promotion of Co/SiO2 Fischer-Tropsch catalysts by niobia and platinum. ACS Catal 6(3):1616–1623. https://doi.org/10.1021/acscatal.5b02418
Liu C, Liu H, Wang B, Hong J, Zhao F, Sun F, Jin S, Liu C, Zhang Y, Li J (2019) Plasma assisted preparation of CoPt/SiO2 Fischer-Tropsch catalysts: a comparison of the precursor pre-thermal treated temperatures. Energy Technol 7(2):224–232. https://doi.org/10.1002/ente.201800669
Jermwongratanachai T, Jacobs G, Ma W, Shafer WD, Gnanamani MK, Gao P, Kitiyanan B, Davis BH, Klettlinger JLS, Yen CH, Cronauer DC, Kropf AJ, Marshall CL (2013) Fischer-Tropsch synthesis: comparisons between Pt and Ag promoted Co/Al2O3 catalysts for reducibility, local atomic structure, catalytic activity, and oxidation–reduction (OR) cycles. Appl Catal A Gen 464–465:165–180. https://doi.org/10.1016/j.apcata.2013.05.040
Sápi A, Rajkumar T, Ábel M, Efremova A, Grósz A, Gyuris A, Ábrahámné KB, Szenti I, Kiss J, Varga T, Kukovecz Á, Kónya Z (2019) Noble-metal-free and Pt nanoparticles-loaded, mesoporous oxides as efficient catalysts for CO2 hydrogenation and dry reforming with methane. J CO2 Util 32:106–118. https://doi.org/10.1016/j.jcou.2019.04.004
Hong J, Du J, Wang B, Zhang Y, Liu C, Xiong H, Sun F, Chen S, Li J (2018) Plasma-assisted preparation of highly dispersed cobalt catalysts for enhanced Fischer-Tropsch synthesis performance. ACS Catal 8(7):6177–6185. https://doi.org/10.1021/acscatal.8b00960
Wang S, Yin Q, Guo J, Ru B, Zhu L (2013) Improved Fischer-Tropsch synthesis for gasoline over Ru, Ni promoted Co/HZSM-5 catalysts. Fuel 108:597–603. https://doi.org/10.1016/j.fuel.2013.02.021
Jacobs G, Ji Y, Davis BH, Cronauer D, Kropf AJ, Marshall CL (2007) Fischer-Tropsch synthesis: temperature programmed EXAFS/XANES investigation of the influence of support type, cobalt loading, and noble metal promoter addition to the reduction behavior of cobalt oxide particles. Appl Catal A Gen 333(2):177–191. https://doi.org/10.1016/j.apcata.2007.07.027
Cook KM, Poudyal S, Miller JT, Bartholomew CH, Hecker WC (2012) Reducibility of alumina-supported cobalt Fischer-Tropsch catalysts: effects of noble metal type, distribution, retention, chemical state, bonding, and influence on cobalt crystallite size. Appl Catal A Gen 449:69–80. https://doi.org/10.1016/j.apcata.2012.09.032
Parnian MJ, Taheri Najafabadi A, Mortazavi Y, Khodadadi AA, Nazzari I (2014) Ru promoted cobalt catalyst on γ-Al2O3: influence of different catalyst preparation method and Ru loadings on Fischer-Tropsch reaction and kinetics. Appl Surf Sci 313:183–195. https://doi.org/10.1016/j.apsusc.2014.05.183
Ma W, Jacobs G, Keogh RA, Bukur DB, Davis BH (2012) Fischer-Tropsch synthesis: effect of Pd, Pt, Re, and Ru noble metal promoters on the activity and selectivity of a 25%Co/Al2O3 catalyst. Appl Catal A Gen 437–438:1–9. https://doi.org/10.1016/j.apcata.2012.05.037
Shariati J, Haghtalab A, Mosayebi A (2019) Fischer-Tropsch synthesis using Co and Co-Ru bifunctional nanocatalyst supported on carbon nanotube prepared via chemical reduction method. J Energy Chem 28:9–22. https://doi.org/10.1016/j.jechem.2017.10.001
Xu R, Hou C, Xia G, Sun X, Li M, Nie H, Li D (2019) Effects of Ag promotion for Co/Al2O3 catalyst in Fischer-Tropsch synthesis. Catal Today. https://doi.org/10.1016/j.cattod.2019.04.004
Dehvari M, Saravani H, Akbarzadeh-T N, Yazdan-Abad MZ (2019) A simple and clean method for the synthesis of Pd/G catalyst for ethanol oxidation. Int J Hydrogen Energy 44(13):6544–6550. https://doi.org/10.1016/j.ijhydene.2019.01.159
Puzan AN, Baumer VN, Mateychenko PV (2017) Structure and decomposition of the silver formate Ag(HCO2). J Solid State Chem 246:264–268. https://doi.org/10.1016/j.jssc.2016.11.022
Khimchenko YI, Vasilenko VP, Radkevich LS, Myalkovskii VV, Chubar TV, Chegoryan VM (1977) Decomposition of iron, cobalt, nickel, and copper formates. Soviet Powder Metall Met Ceram 16(5):327–332. https://doi.org/10.1007/BF00791078
Qusti A, Samarkandy AA, Al-Thabaiti SA, Diefallah E-HM (1997) The kinetics of thermal decomposition of nickel formate dihydrate in air. J King Saud Univ Sci 9:73–81. https://doi.org/10.4197/Sci.9-1.7
Won HI, Nersisyan H, Won CW, Lee J-M, Hwang J-S (2010) Preparation of porous silver particles using ammonium formate and its formation mechanism. Chem Eng J 156(2):459–464. https://doi.org/10.1016/j.cej.2009.10.053
Rosen Y, Marrach R, Gutkin V, Magdassi S (2019) Thin copper flakes for conductive inks prepared by decomposition of copper formate and ultrafine wet milling. Adv Mater Technol 4(1):1800426. https://doi.org/10.1002/admt.201800426
Rosenband V, Gany A (2004) Preparation of nickel and copper submicrometer particles by pyrolysis of their formates. J Mater Process Technol 153–154:1058–1061. https://doi.org/10.1016/j.jmatprotec.2004.04.165
Harmel J, Peres L, Estrader M, Berliet A, Maury S, Fécant A, Chaudret B, Serp P, Soulantica K (2018) hcp-Co nanowires grown on metallic foams as catalysts for Fischer-Tropsch synthesis. Angew Chem Int Ed 57(33):10579–10583. https://doi.org/10.1002/anie.201804932
Gao S, Hong J, Xiao G, Chen S, Zhang Y, Li J (2019) Evolution of cobalt species in glow discharge plasma prepared CoRu/SiO2 catalysts with enhanced Fischer-Tropsch synthesis performance. J Catal 374:246–256. https://doi.org/10.1016/j.jcat.2019.04.039
Gavrilović L, Brandin J, Holmen A, Venvik HJ, Myrstad R, Blekkan EA (2018) Fischer-Tropsch synthesis—investigation of the deactivation of a Co catalyst by exposure to aerosol particles of potassium salt. Appl Catal B Environ 230:203–209. https://doi.org/10.1016/j.apcatb.2018.02.048
Bae J-S, Hong SY, Park JC, Rhim GB, Youn MH, Jeong H, Kang SW, Yang J-I, Jung H, Chun DH (2019) Eco-friendly prepared iron-ore-based catalysts for Fischer-Tropsch synthesis. Appl Catal B Environ 244:576–582. https://doi.org/10.1016/j.apcatb.2018.11.082
Khodakov AY (2009) Fischer-Tropsch synthesis: relations between structure of cobalt catalysts and their catalytic performance. Catal Today 144(3):251–257. https://doi.org/10.1016/j.cattod.2008.10.036
Shi L, Tao K, Kawabata T, Shimamura T, Zhang XJ, Tsubaki N (2011) Surface impregnation combustion method to prepare nanostructured metallic catalysts without further reduction: As-burnt Co/SiO2 catalysts for Fischer-Tropsch Synthesis. ACS Catal 1(10):1225–1233. https://doi.org/10.1021/cs200294d
Shi L, Jin Y, Xing C, Zeng C, Kawabata T, Imai K, Matsuda K, Tan Y, Tsubaki N (2012) Studies on surface impregnation combustion method to prepare supported Co/SiO2 catalysts and its application for Fischer-Tropsch synthesis. Appl Catal A Gen 435–436:217–224. https://doi.org/10.1016/j.apcata.2012.06.007
Shi L, Zeng C, Lin Q, Lu P, Niu W, Tsubaki N (2014) Citric acid assisted one-step synthesis of highly dispersed metallic Co/SiO2 without further reduction: as-prepared Co/SiO2 catalysts for Fischer-Tropsch synthesis. Catal Today 228:206–211. https://doi.org/10.1016/j.cattod.2013.10.013
Pengnarapat S, Ai P, Reubroycharoen P, Vitidsant T, Yoneyama Y, Tsubaki N (2018) Active Fischer-Tropsch synthesis Fe-Cu-K/SiO2 catalysts prepared by autocombustion method without a reduction step. J Energy Chem 27(2):432–438. https://doi.org/10.1016/j.jechem.2017.11.029
Xiong H, Moyo M, Rayner MK, Jewell LL, Billing DG, Coville NJ (2010) Autoreduction and catalytic performance of a cobalt Fischer-Tropsch synthesis catalyst supported on nitrogen-doped carbon spheres. ChemCatChem 2(5):514–518. https://doi.org/10.1002/cctc.200900309
Yang Y, Jia L, Hou B, Li D, Wang J, Sun Y (2014) The effect of nitrogen on the autoreduction of cobalt nanoparticles supported on nitrogen-doped ordered mesoporous carbon for the Fischer-Tropsch synthesis. ChemCatChem 6(1):319–327. https://doi.org/10.1002/cctc.201300897
Park H, Kim KY, Youn DH, Choi YH, Kim WY, Lee JS (2017) Auto-reduction behavior of cobalt on graphitic carbon nitride coated alumina supports for Fischer-Tropsch synthesis. ChemCatChem 9(21):4098–4104. https://doi.org/10.1002/cctc.201700613
Lü J, Huang C, Bai S, Jiang Y, Li Z (2012) Thermal decomposition and cobalt species transformation of carbon nanotubes supported cobalt catalyst for Fischer-Tropsch synthesis. J Nat Gas Chem 21(1):37–42. https://doi.org/10.1016/S1003-9953(11)60330-7
Ni Z, Zhang X, Bai J, Wang Z, Li X, Zhang Y (2020) Potassium promoted core–shell-structured FeK@SiO2-GC catalysts used for Fischer-Tropsch synthesis to olefins without further reduction. New J Chem 44(1):87–94. https://doi.org/10.1039/C9NJ03947C
Dalai AK, Davis BH (2008) Fischer-Tropsch synthesis: a review of water effects on the performances of unsupported and supported Co catalysts. Appl Catal A Gen 348(1):1–15. https://doi.org/10.1016/j.apcata.2008.06.021
Arsalanfar M, Fatemi M, Mirzaei N, Abdouss M, Rezazadeh E (2020) Study of mechanism and kinetic modeling of CO hydrogenation reaction over the impregnated Co-Ni/Al2O3 catalyst. J Chin Chem Soc 67(7):1152–1166. https://doi.org/10.1002/jccs.201900526
Escalante M, Maury P, Bruinink CM, van der Werf K, Olsen JD, Timney JA, Huskens J, Neil Hunter C, Subramaniam V, Otto C (2007) Directed assembly of functional light harvesting antenna complexes onto chemically patterned surfaces. Nanotechnology 19(2):025101. https://doi.org/10.1088/0957-4484/19/02/025101
Taherzadeh Lari T, Mirzaei AA, Atashi H (2017) Effects of Co/Ce molar ratio and operating temperature on nanocatalyst performance in the Fischer-Tropsch synthesis. RSC Adv 7(55):34497–34507. https://doi.org/10.1039/C7RA05490D
Haghtalab A, Shariati J, Mosayebi A (2019) Experimental and kinetic modeling of Fischer-Tropsch synthesis over nano structure catalyst of Co–Ru/carbon nanotube. Reac Kinet Mech Cat 126(2):1003–1026. https://doi.org/10.1007/s11144-019-01535-7
Acknowledgements
The authors gratefully appreciate University of Sistan and Baluchestan for helping and supporting this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eshraghi, A., Mirzaei, A.A., Rahimi, R. et al. A simple and low cost method for the synthesis of metallic cobalt nanoparticles without further reduction as an effective catalyst for Fischer–Tropsch Synthesis. Reac Kinet Mech Cat 134, 127–141 (2021). https://doi.org/10.1007/s11144-021-02046-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02046-0