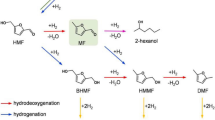
Abstract
In this report, promising carbon-based novel metal phthalocyanine catalysts were prepared and tested in glucose dehydration at low temperatures (90–130 °C) under atmospheric conditions. Most of the prepared phthalocyanines exhibited high conversions in dimethylsulfoxide (DMSO). A complete conversion of glucose was obtained over Aluminum phthalocyanine (AlPcCl) and Tin phthalocyanine (SnPcCl2) catalysts. AlPcCl also provided the highest HMF yield (53% in 3 h at 130 °C). Al3+ cations promoted the glucose conversion and Cl− anions played an active role in 5-Hydroxymethylfurfural (5-HMF) formation. Dimethylsulfoxide (DMSO) as a solvent, was also found effective with phthalocyanines for high 5-HMF yield by stabilizing its structure. Thus, the 5-HMF yield was observed high (53.0% at 130 °C for 3 h) over AlPcCl in DMSO. Kinetic studies showed that the optimum reaction temperature was 130 °C at 1/5 catalyst/glucose ratio for 3 h. Dehydration reactions, performed in the aqueous phase, exhibited higher fructose selectivities with AlPcCl catalyst.
Graphic abstract







Similar content being viewed by others
References
Gürbüz E, Bond JQ, Dumesic JA, Román-Leshkov Y (2013) Role of acid catalysis in the conversion of lignocellulosic biomass to fuels and chemicals. Role Catal Sustain Prod Bio-Fuels Bio-Chem. https://doi.org/10.1016/B978-0-444-56330-9.00008-5
Martínez CM, Cantero DA, Cocero MJ (2018) Production of saccharides from sugar beet pulp by ultrafast hydrolysis in supercritical water. J Clean Prod 204:888–895. https://doi.org/10.1016/j.jclepro.2018.09.066
Sahu R, Dhepe PL (2014) Synthesis of 2,5-furandicarboxylic acid by the aerobic oxidation of 5-hydroxymethyl furfural over supported metal catalysts. React Kinet Mech Catal 112:173–187. https://doi.org/10.1007/s11144-014-0689-z
Fan Y, Zhu M, Jin L et al (2020) Catalytic upgrading of biomass-derived vapors to bio-fuels via modified HZSM-5 coupled with DBD: effects of different titanium sources. Renew Energy 157:100–115. https://doi.org/10.1016/j.renene.2020.05.019
Wang Y, Deng W, Wang B et al (2013) Chemical synthesis of lactic acid from cellulose catalysed by lead(II) ions in water. Nat Commun 4:1–7. https://doi.org/10.1038/ncomms3141
Khayoon MS, Hameed BH (2011) Acetylation of glycerol to biofuel additives over sulfated activated carbon catalyst. Bioresour Technol 102:9229–9235. https://doi.org/10.1016/j.biortech.2011.07.035
Abad-Fernández N, Pérez E, Martín Á, Cocero MJ (2020) Kraft lignin depolymerisation in sub- and supercritical water using ultrafast continuous reactors optimization and reaction kinetics. J Supercrit Fluids. https://doi.org/10.1016/j.supflu.2020.104940
Abdouli I, Eternot M, Dappozze F et al (2020) Comparison of hydrothermal and photocatalytic conversion of glucose with commercial TiO2: superficial properties-activities relationships. Catal Today. https://doi.org/10.1016/j.cattod.2020.03.040
Hu L, Lin L, Wu Z et al (2015) Chemocatalytic hydrolysis of cellulose into glucose over solid acid catalysts. Appl Catal B 174–175:225–243. https://doi.org/10.1016/j.apcatb.2015.03.003
Ji J, Xu Y, Liu Y, Zhang Y (2020) A nanosheet Ru/WO3 catalyst for efficient conversion of glucose to butanediol. Catal Commun 144:106074. https://doi.org/10.1016/j.catcom.2020.106074
Xu J, Li L, Li G et al (2018) Synthesis of renewable C8–C10 alkanes with angelica lactone and furfural from carbohydrates. ACS Sustain Chem Eng 6:6126–6134. https://doi.org/10.1021/acssuschemeng.7b04797
Nikolla E, Román-Leshkov Y, Moliner M, Davis ME (2011) “One-pot” synthesis of 5-(hydroxymethyl)furfural from carbohydrates using tin-beta zeolite. ACS Catal 1:408–410. https://doi.org/10.1021/cs2000544
Xu J, Li N, Yang X et al (2017) Synthesis of diesel and jet fuel range alkanes with furfural and angelica lactone. ACS Catal 7:5880–5886. https://doi.org/10.1021/acscatal.7b01992
Galebach PH, McClelland DJ, Eagan NM et al (2018) Production of alcohols from cellulose by supercritical methanol depolymerization and hydrodeoxygenation. ACS Sustain Chem Eng 6:4330–4344. https://doi.org/10.1021/acssuschemeng.7b04820
Käldström M, Kumar N, Murzin DY (2011) Valorization of cellulose over metal supported mesoporous materials. Catal Today 167:91–95. https://doi.org/10.1016/j.cattod.2010.12.048
Geboers J, Van de Vyver S, Carpentier K et al (2011) Efficient hydrolytic hydrogenation of cellulose in the presence of Ru-loaded zeolites and trace amounts of mineral acid. Chem Commun 47:5590–5592. https://doi.org/10.1039/c1cc10422e
Meng Q, Hou M, Liu H et al (2017) Synthesis of ketones from biomass-derived feedstock. Nat Commun. https://doi.org/10.1038/ncomms14190
Zhang X, Hewetson BB, Mosier NS (2015) Kinetics of maleic acid and aluminum chloride catalyzed dehydration and degradation of glucose. Energy Fuels 29:2387–2393. https://doi.org/10.1021/ef502461s
Son PA, Nishimura S, Ebitani K (2014) Production of γ-valerolactone from biomass-derived compounds using formic acid as a hydrogen source over supported metal catalysts in water solvent. RSC Adv 4:10525–10530. https://doi.org/10.1039/c3ra47580h
Trapp T, Kirchner T, Birk F et al (2019) Biosynthesis of stereoisomers of dill ether and wine lactone by Pleurotus sapidus. J Agric Food Chem 67:13400–13411. https://doi.org/10.1021/acs.jafc.8b07263
Wang F, Wang Y, Jin F et al (2014) One-pot hydrothermal conversion of cellulose into organic acids with cuo as an oxidant. Ind Eng Chem Res 53:7939–7946. https://doi.org/10.1021/ie404311d
Hu S, Smith TJ, Lou W, Zong M (2014) Efficient hydrolysis of cellulose over a novel sucralose-derived solid acid with cellulose-binding and catalytic sites. J Agric Food Chem 62:1905–1911. https://doi.org/10.1021/jf405712b
Pagán-Torres YJ, Wang T, Gallo JMR et al (2012) Production of 5-hydroxymethylfurfural from glucose using a combination of lewis and brønsted acid catalysts in water in a biphasic reactor with an alkylphenol solvent. ACS Catal 2:930–934. https://doi.org/10.1021/cs300192z
Tyagi U, Anand N (2020) Single-pot conversion of fruit peel waste to 5-hydroxymethylfurfural catalyzed by modified activated carbon in green solvent: kinetics and thermodynamic study. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00811-0
Marianou AA, Michailof CM, Pineda A et al (2018) Effect of Lewis and Br Ønsted acidity on glucose conversion to 5-HMF and lactic acid in aqueous and organic media. Appl Catal A 555:75–87. https://doi.org/10.1016/j.apcata.2018.01.029
de Melo FC, Bariviera W, Zanchet L et al (2020) C10MI·CF3SO3: a hydrophobic ionic liquid medium for the production of HMF from sugars avoiding the use of organic solvent. Biomass Convers Biorefinery 10:611–618. https://doi.org/10.1007/s13399-019-00446-w
Garcés D, Díaz E, Ordóñez S (2017) Aqueous phase conversion of hexoses into 5-hydroxymethylfurfural and levulinic acid in the presence of hydrochloric acid: mechanism and kinetics. Ind Eng Chem Res 56:5221–5230. https://doi.org/10.1021/acs.iecr.7b00952
Babaei Z, Yazdanpanah Esmaeilabad R, Orash N, Najafi Chermahini A (2020) Sulfonated CMK-3: an effective catalyst for the glucose conversion to butyl levulinate as the fuel additive. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-01072-7
Saravanan K, Park KS, Jeon S, Bae JW (2018) Aqueous phase synthesis of 5-hydroxymethylfurfural from glucose over large pore mesoporous zirconium phosphates: effect of calcination temperature. ACS Omega 3:808–820. https://doi.org/10.1021/acsomega.7b01357
Thunyaratchatanon C, Sinsakullert W, Luengnaruemitchai A, Faungnawakij K (2019) 5-Hydroxymethylfurfural production from hexose sugars using adjustable acid- and base-functionalized mesoporous SBA-15 catalysts in aqueous media. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-019-00553-8
Hoşgün HL, Türe AG, Hoşgün EZ, Bozan B (2020) Synthesis 5-hydroxymethylfurfural (5-HMF) from fructose over cetyl trimethylammonium bromide-directed mesoporous alumina catalyst: effect of cetyl trimethylammonium bromide amount and calcination temperature. React Kinet Mech Catal 129:337–347. https://doi.org/10.1007/s11144-019-01699-2
Shiwei W, Qibao W (2018) Selective conversion of glucose into lactic acid with immobilized ytterbium triflate. React Kinet Mech Catal 125:923–936. https://doi.org/10.1007/s11144-018-1448-3
Isaeva VI, Nefedov OM, Kustov LM (2018) Metal–organic frameworks-based catalysts for biomass processing. Catalysts. https://doi.org/10.3390/catal8090368
Dosuna-Rodríguez I, Adriany C, Gaigneaux EM (2011) Glycerol acetylation on sulphated zirconia in mild conditions. Catal Today 167:56–63. https://doi.org/10.1016/j.cattod.2010.11.057
Oozeerally R, Burnett DL, Chamberlain TW et al (2017) Exceptionally efficient and recyclable heterogeneous metal-organic framework catalyst for glucose isomerization in water. ChemCatChem. https://doi.org/10.1002/cctc.201701825
Mangematin S, Sorokin AB (2001) Synthesis and catalytic properties of a novel phthalocyanine covalently grafted onto silica. J Porphyr Phthalocyanines 5:674–680. https://doi.org/10.1002/jpp.379
Shaabani A, Keshipour S, Hamidzad M, Shaabani S (2014) Cobalt(II) phthalocyanine covalently anchored to cellulose as a recoverable and efficient catalyst for the aerobic oxidation of alkyl arenes and alcohols. J Mol Catal A 395:494–499. https://doi.org/10.1016/j.molcata.2014.09.003
Saka ET, Çağlar Y (2017) New Co(II) and Cu(II) phthalocyanine catalysts reinforced by long alkyl chains for the degradation of organic pollutants. Catal Lett 147:1471–1477. https://doi.org/10.1007/s10562-017-2054-0
Yadav KK, Ahmad S, Chauhan SMS (2014) Elucidating the role of cobalt phthalocyanine in the dehydration of carbohydrates in ionic liquids. J Mol Catal A 394:170–176. https://doi.org/10.1016/j.molcata.2014.07.014
Tsilomelekis G, Josephson TR, Nikolakis V, Caratzoulas S (2014) Origin of 5-hydroxymethylfurfural stability in water/dimethyl sulfoxide mixtures. Chemsuschem 7:117–126. https://doi.org/10.1002/cssc.201300786
Owen JE, Kenney ME (1962) Phthalocyaninoaluminum compounds. Inorg Chem 1:331–333. https://doi.org/10.1021/ic50002a026
Pawlovski GMH (1980) Synthetic communications. Synth Commun. https://doi.org/10.1080/00397918008064262
Baygu Y, Kabay N, Gök Y Synthesis and characterization of novel galactose containing phthalocyanines. Unpubl results
Kabay N, Baygu Y, Ak M Kara İ, Kaya EN, Durmuş M, Gök Y Novel octa-nonperipheral 3-hydroxypropylthio substituted metallo-phthalocyanines: synthesis, characterization and investigation of electrochemical, photochemical and computational properties, submitted for publication. (Submitted for publications)
Myers JF, Canham GWR, Lever ABP (1975) Higher oxidation level phthalocyanine complexes of chromium, iron, cobalt and zinc. Phthalocyanine Radical Species Inorg Chem 14:461–468. https://doi.org/10.1021/ic50145a002
Meller A, Ossko A (1972) Phthalocyaninartige Bor-Komplexe-15c-Halogeno-triisoindolo[1,2,3-cd:1′,2′,3′-gh:1″,2″,3″-kl]-[2,3a,5,6a,8,9a,9b]-hexaazaboraphenalene. Monatsh Chem 103:150–155. https://doi.org/10.1007/BF00912939
Kilic A, Ulusoy M, Durgun M, Aytar E (2014) The multinuclear cobaloxime complexes-based catalysts for direct synthesis of cyclic carbonate from of epichlorohydrin using carbon dioxide: synthesis and characterization. Inorganica Chim Acta 411:17–25. https://doi.org/10.1016/j.ica.2013.11.005
Pang J, Wang A, Zheng M, Zhang T (2010) Hydrolysis of cellulose into glucose over carbons sulfonated at elevated temperatures. Chem Commun 46:6935–6937. https://doi.org/10.1039/c0cc02014a
Miessler GL, Fischer PJ (2014) Inorganic chemistry, 5th edn. Pearson Education Inc, New York
Wang T, Glasper JA, Shanks BH (2015) Kinetics of glucose dehydration catalyzed by homogeneous Lewis acidic metal salts in water. Appl Catal A 498:214–221. https://doi.org/10.1016/j.apcata.2015.03.037
Van Zandvoort I, Koers EJ, Weingarth M et al (2015) Structural characterization of 13C-enriched humins and alkali-treated 13C humins by 2D solid-state NMR. Green Chem 17:4383–4392. https://doi.org/10.1039/c5gc00327j
Qian X, Liu D (2014) Free energy landscape for glucose condensation and dehydration reactions in dimethyl sulfoxide and the effects of solvent. Carbohydr Res 388:50–60. https://doi.org/10.1016/j.carres.2014.02.010
Amarasekara AS, Williams LTD, Ebede CC (2008) Mechanism of the dehydration of d-fructose to 5-hydroxymethylfurfural in dimethyl sulfoxide at 150 °C: an NMR study. Carbohydr Res 343:3021–3024. https://doi.org/10.1016/j.carres.2008.09.008
Mellmer MA, Sanpitakseree C, Demir B et al (2019) Effects of chloride ions in acid-catalyzed biomass dehydration reactions in polar aprotic solvents. Nat Commun 10:1–10. https://doi.org/10.1038/s41467-019-09090-4
Lente G (2015) Deterministic kinetics in chemistry and systems biology the dynamics of complex reaction networks. Springer, New York
Rasrendra CB, Soetedjo JNM, Makertihartha IGBN et al (2012) The catalytic conversion of D-glucose to 5-hydroxymethylfurfural in DMSO using metal salts. Top Catal 55:543–549. https://doi.org/10.1007/s11244-012-9826-y
Oozeerally R, Ramkhelawan SDK, Burnett DL et al (2019) ZIF-8 metal organic framework for the conversion of glucose to fructose and 5-hydroxymethyl furfural. Catalysts. https://doi.org/10.3390/catal9100812
Acknowledgements
This work was mainly financially supported by the Research Fund of Usak University (2019/DTS005) and the Regional Development Program.
Funding
This work was mainly financially supported by the Research Fund of Usak University (2019/DTS005) and Regional Development Program.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have approved the final version of the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kılıç, H.A., Kılıç, E., Kabay, N. et al. Selective glucose dehydration over novel metal phthalocyanine catalysts at low temperatures. Reac Kinet Mech Cat 132, 637–654 (2021). https://doi.org/10.1007/s11144-021-01969-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-01969-y