Abstract
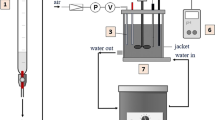
Noncatalytic isomerization (R1) and hydration (R2) of fumaric acid (F) was carried out homogeneously and isothermally in an electric-heated batch reactor (reactor 1), in a microwave-heated tank reactor (reactor 2), and in an oven-heated tubular reactor (reactor 3) at temperatures between 448 and 623 K. The concentrations of F, malic acid (M), and maleic acid (Mx) were determined chromatographically at reaction times from zero up to 25,200 s. The variation in the rate constants for the forward reactions R1 and R2 with temperature was estimated with an Arrhenius type equation by involving parameters tuned on the results obtained in the first reactor (k10,α = 1.08×10−1 s−1, Ea1,α/R = 5070 K, k20,α = 1.62×101 M−1 s−1, Ea2,α/R = 8331 K). The set of data from the experiments performed in the reactors 2 and 3 under a variety of operating conditions was used to validate the tuned kinetic parameters. To investigate the contribution of the noncatalytic consumption of F to the overall conversion with hydrochloric acid as catalyst, an available kinetic model that takes the concentration of HCl into account was modified. The old model, which was only able to estimate correctly the kinetics of acid-catalyzed conversion of F with HCl at concentrations higher than 0.65 M, can now describe the kinetics of the same reaction system properly without HCl, or at any amount of this catalyst.







Similar content being viewed by others
Abbreviations
- C F :
-
Concentration of fumaric acid (mol l−1)
- C F0 :
-
Initial concentration of fumaric acid (mol l−1)
- C H :
-
Concentration of hydrochloric acid (mol l−1)
- C M :
-
Concentration of malic acid (mol l−1)
- C Mx :
-
Concentration of maleic acid (mol l−1)
- C W :
-
Concentration of water (mol l−1)
- E a1,α :
-
Tuned activation energy of the isomerization reaction without HCl (J kmol−1)
- E a1,β :
-
Tuned activation energy of the isomerization reaction with HCl (J kmol−1)
- E a2,α :
-
Tuned activation energy of the hydration reaction without HCl (J kmol−1)
- E a2,β :
-
Tuned activation energy of the hydration reaction with HCl (J kmol−1)
- f :
-
Objective function defined by Eq. 7
- k 1,α :
-
Forward-rate constant of the isomerization reaction R1 without HCl (s−1)
- k 1,β :
-
Forward-rate constant of the isomerization reaction R1 with HCl (M−1 s−1)
- k 10,α :
-
Tuned preexponential factor of the isomerization reaction R1 without HCl (s−1)
- k 10,β :
-
Tuned preexponential factor of the isomerization reaction R1 with HCl (M−1 s−1)
- K 1 :
-
Equilibrium constant for the isomerization reaction R1 at a temperature T
- k 2,α :
-
Forward-rate constant of the hydration reaction R2 without HCl (M−1 s−1)
- k 2,β :
-
Forward-rate constant of the hydration reaction R2 with HCl (M−2 s−1)
- k 20,α :
-
Tuned preexponential factor of the hydration reaction R2 without HCl (M−1 s−1)
- k 20,β :
-
Tuned preexponential factor of the hydration reaction R2 with HCl (M−2 s−1)
- K 2 :
-
Equilibrium constant for the hydration reaction R2 at a temperature T (M−1)
- P :
-
Reaction pressure (kPa)
- R :
-
Gas constant (J kmol−1 K−1)
- S :
-
Ratio between the concentration of malic and maleic acid, CM/CMx
- t :
-
Reaction time (s)
- T :
-
Reaction temperature (K)
- X F :
-
Overall conversion of fumaric acid
References
Francis FJ (1999) Wiley encyclopedia of food science and technology. Wiley, Hoboken
Kirk RE, Othmer DF (2007) Encyclopedia of chemical technology, vol 13 and 15. Wiley, Hoboken
Huang J, Huang L, Lin J et al (2010) Organic chemicals from bioprocesses in China. Adv Biochem Eng Biotechnol 122:43–71
Grand View Research (2014) Malic acid market analysis by application (beverages, confectionary & food) and segment forecast to 2020. Grand View Research Inc., San Francisco
Lee BH (2015) Fundamentals of food biotechnology. Wiley, Hoboken
Wang B, Li H, Zhu L et al (2017) High-efficient production of citric acid by Aspergillus niger from high concentration of substrate based on the staged-addition glucoamylase strategy. Bioproc Biosyst Eng 40:891–899
Arslan NP, Aydogan MN, Taskin M (2016) Citric acid production from partly deproteinized whey undernon-sterile culture conditions using immobilized cells oflactose—positive and cold-adapted Yarrowia lipolytica B9. J Biotechnol 231:32–39
Ortiz RWP, Benincá C, Cardozo-Filho L, Zanoelo EF (2017) High-pressure acid-catalyzed isomerization and hydration of fumaric acid in a homogeneous nonisothermal batch reactor. Ind Eng Chem Res 56:3873–3879
Ortiz RWP, de Jesus BG, Franceschi E et al (2017) Microwave-assisted synthesis of malic acid involving hydrochloric acid as catalyst. Reac Kinet Mech Cat 122:793–802
Rozelle LT, Alberty RA (1957) Kinetics of the acid catalysis of the hydration of fumaric acid to malic acid. J Phys Chem 61:1637–1640
Bender ML, Connors KA (1962) A non-enzymatic olefinic hydration under neutral conditions: the kinetics and mechanism of the hydration of fumaric acid monoanion. J Am Chem Soc 84:1980–1986
Bada JL, Miller SL (1969) The kinetics of hydration of fumaric acid between pH 0 and 6. J Am Chem Soc 91:3948–3949
Goldberg I, Williams R (1991) Biotechnology and food ingredients. Van Nostrand Reinhold, New York
Weinrotter F, Schmidt A, Gauster W (1975) Process for the production of malic acid. US Patent 3875222
Zelle RM, Hulster E, van Winden WA et al (2008) Malic acid production by Saccharomyces cerevisiae: engineering of pyruvate carboxylation, oxaloacetate reduction and malate export. Appl Environ Microb 74:2766–2777
Ramsey SH, Schultz RG (1993) Preparation of malic acid. US Patent 5210295
Tálas E, Szíjjártó GP, Tompos A (2015) Hydroamination reactions of dialkyl esters of 2- buthenedioic acids with polyetheramines under catalytic and non-catalytic conditions. Reac Kinet Mech Cat 115:431–447
Shi H, Zhou Y, Zuo Y et al (2017) Heterogeneous catalysis of CO2-diethanolamine absorption with MgCO3 and CaCO3 and comparing to non-catalytic CO2-monoethanolamine interactions. Reac Kinet Mech Cat 122:539–555
Ferreira-Pinto L, Feirhrmann AC, Corazza ML et al (2015) Hydrogen production and TOC reduction from gasification of lactose by supercritical water. Int J Hydrog Energy 40:12162–12168
AOAC (1990) Food compositions; additives, natural contaminants (Official Method AOAC 986.13: quinic, malic, citric acid in cranberry juice cocktail and apple juice). AOAC, Arlington
Petzold LRA (1982) Description of DASSL: a differential/algebraic system solver. Report SAND82-8637, Sandia, Albuquerque
Kanda LRS, Corazza ML, Zatta L, Wypych F (2017) Kinetics evaluation of the ethyl esterification of long chain fatty acids using commercial montmorillonite K10 as catalyst. Fuel 193:265–274
Alenezi R, Leeke GA, Winterbottom JM et al (2010) Esterification kinetics of free fatty acids with supercritical methanol for biodiesel production. Energy Convers Manag 51:1055–1059
Echaroj S, Santikunaporn M, Chavadej S (2015) Micro-kinetic modeling of the catalytic dehydration of 1-decanol over precipitated γ-Al2O3. Reac Kinet Mech Cat 114:75–91
Chun Y, He YG, Zhu JH (2001) Microwave-assisted synthesis of dimethyl carbonate. React Kinet Catal Lett 74:23–27
Rosana MR, Hunt J, Ferrari A et al (2014) Microwave-specific acceleration of a Friedel-Crafts reaction: evidence for selective heating in homogeneous solution. J Org Chem 79:7437–7450
Priebe JM, Dall’Oglio EL, de Sousa Jr PT et al (2016) Measurements of physicochemical properties during microwave-assisted acid-catalyzed transesterification reactions. Energy Fuels 30:3066–3077
Savage PE (2009) A perspective on catalysis in sub- and supercritical water. J Supercrit Fluid 47:407–414
Galkin AA, Lunin VV (2005) Subcritical and supercritical water: a universal medium for chemical reactions. Russ Chem Rev 74:21–35
Acknowledgements
Prof. Marcos Corazza and Prof. Fernando A. P. Voll are gratefully acknowledged for making available the facilities of the LACTA laboratory for this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mattar Knesebeck, A., Ortiz, R.W.P., Cardozo-Filho, L. et al. Isomerization and hydration of fumaric acid under catalytic and noncatalytic conditions. Reac Kinet Mech Cat 125, 521–534 (2018). https://doi.org/10.1007/s11144-018-1458-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-018-1458-1