Abstract
The kinetics of Pd(II) chloride complex ion recovery from diluted aqueous solutions was investigated using commercially available activated carbon. For this purpose, several different parameters as the influence of temperature, mixing rate, amount of activated carbon applied as well as initial concentrations of Pd(II) chloride complex ions were analyzed. The obtained results showed that the rate of the process is controlled by the mass transfer from the solution to the surface of AC. Therefore, a significant impact of the mixing rate was observed. The activation energy of each step was determined and it is found to be 23.5 ± 1.3, 52.7 ± 5.5 and 22.8 ± 9.5 kJ/mol for adsorption, desorption and reaction of Pd(II) chloride complex transformation. It was shown that commercially available activated carbon, initially designed for gas purification, can be also used for metal ions removal during water purification as well as for precious metal recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Palladium is one of the most important precious metals. It belongs to the platinum group metals (PGMs) which taking into account its availability is called critical metal group. In the case of palladium, about 79% of world’s production originates from countries like Russia and South Africa. Due to the high price, recycling of these elements is highly developed. For precious metal recovery, mainly hydrometallurgical methods are applied. It is due to the fact that not very large quantities of metal are processed in most cases. For example in the case of gold, hydrometallurgical methods are mainly used. Especially, cyanide leaching and cyanide complexes adsorption using activated carbon is one of the most often applied method for its preconcentration [1,2,3,4]. In the case of platinum, adsorption processes can be also applied [5,6,7,8].
Processes of different metal ion adsorptions were investigated and described in a large number of papers. Adsorption processes are mainly recommended for water purification as well as drug purifications. In the case of palladium, it seems that the adsorption processes might be also used. However, there is a limited number of papers dealing with the kinetic studies of adsorption of palladium on different sort of activated carbons.
Therefore, in this paper, the results related to the adsorption of Pd(II) complexes on commercially activated carbon are given.
Experimental
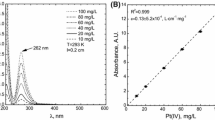
The decay of the concentration of Pd(II) chloride complex ions in the solutions during adsorption experiments was monitored spectrophotometrically (Shimadzu model U-2501PC). For this purpose, a portion of 1 or 4 mL of the Pd(II) solution was introduced into the quartz cuvette and then the UV–VIS spectrum was recorded. The path length of the cuvette was varied depending on the Pd(II) concentration in the solution (from 0.2 to 1 cm). Therefore, different portions of the analyzed solutions were required depending on cuvette size. After analysis, the sample was returned to the reactor. All UV–VIS spectra were recorded at the temperature of 293 K. For this purpose, samples were required to be cooled down. The Shimadzu (model U-2501PC) spectrophotometer was equipped with suitable module (Peltier cell).
The concentration of Pd(II) in the solution can be calculated from the UV–VIS spectrum taking into account the Beer law (see Eq. 1)
Here abs is the absorbance, A.U; \(\varepsilon\) is the molar absorption coefficient, \(\left[ {\frac{{\rm dm^{3} }}{\rm cm \times mol}} \right]\); l is the path length, cm.
Knowing a molar absorption coefficient for Pd(II) as well as absorbance level it is possible to calculate concentration of Pd(II). It is known that molar absorption coefficient for Pd(II) ions at pH = 1 is equal to 6961.7 ± 14 [9, 10].
The BET analysis as well as mercury porosimetry of the activated carbon (AC) were performed and the results were shown in the previous paper [11]. Primarily, AC ROX 0.8 was designed for gas purification. In this paper, this AC was used for heavy metal removal. In all experiments, AC without purification and any chemical modification was applied. Carbon has a shape of rods with the diameter of 0.8 mm. Length of the rod is different and varies from 2 up to 10 mm.
All kinetic experiments were carried out using 0.3 dm3 portions of the solution containing Pd(II) ions. The amount of AC was varied in the rage from 0.33 to 6.67 g/L. pH of the solution was equal to 1 (unless otherwise stated), and was controlled by dissolution of required amount of Pd(II) stock solution in 0.1 M of hydrochloric acid. A solution of 0.1 M hydrochloric acid was obtained by the dissolution of a suitable amount of concentrated hydrochloric acid (37%, Avantor, analytical grade).
The Pd(II) stock solution was prepared in accordance with our previous study [12]. Solutions were agitated with a mechanical stirrer (Cat R50). The stirrer was made of glass to prevent Pd(II) reduction or adsorption on the surface of a stirrer. Experiments were carried out in thermostated cyclic reactor. The temperature was varied in the rage from 298 to 348 K. During the experiment, the temperature was monitored and kept constant ± 0.5 °C.
The XPS analysis of the adsorbent before and after sorption experiments was carried out (SES R4000 equipped with hemispherical analyzer).
SEM analysis was performed using SEM (scanning electron microscopy technique) Hitachi SU-70 equipped with EDS, WDS and EBSD add-on devices (Thermo Scientific).
Results
XPS analysis of AC after and before adsorption process
In Fig. 1, a survey spectrum of the sample of activated carbon after adsorption of Pd(II) ions is shown. It can be seen that palladium is present in the sample after adsorption.
Moreover, it can be seen that there is two times more chlorine than palladium. This suggests that formed on the surface species have stoichiometry 2–1.
However, to make sure, additional experiments are required. The detailed analysis of the palladium sample brings the information about electronic state of this element. This result is shown in Fig. 2.
From the obtained Pd3d core excitation data, it is possible to conclude that either 100% of Pd exists on the surface in the metallic form (but in the nano scale) or palladium is present on the surface in the form of Pd2+ strongly bonded with the organic molecules (e.g. oxygen containing functional groups). In the case of nano Pd particles, a shift similar to Pd(II) in binding energy is observed. To clarify these doubts, further analysis was perform using the SEM technique. That analysis should confirm if Pd2+ is linked to Cl−.
SEM analysis of AC surface after adsorption of Pd(II)
In Fig. 3, the SEM image of cross section of AC after adsorption of Pd(II) is shown. It is known that in the presence of metallic Pd on the surface, it should be possible to see it as bright spots.
In the studied case, no metallic form was observed. Composition analysis along the line shown as a cross-section of AC is given in Fig. 3c (line consist of 800 measurement points). As it can be seen, significant fluctuation in the signal intensity can be observed. This is related to the roughness of AC cross-section and its porosity. However, it can be observed that an increase of signal coming from Pd is also parallel to the increase of signal coming from Cl. This can be taken as the confirmation that Pd on the surface of AC is in the form of PdCl2, as it was suggested directly after XPS analysis.
Kinetic curve analysis
As it was mentioned in the experimental section, changes in the concentration of Pd(II) ions in the solution during the adsorption process were monitored spectrophotometrically. Fig. 4 shows an example of a kinetic curve registered during adsorption tests.
The registered kinetic curve indicates a gradual decrease with time, which can be described by the general equation:
Here \([Pd(II)]_{t}\)- concentration of Pd(II) complex in time, W1, W2, and γ1, γ2 are fitted parameters.
As it can be observed, Eq. 2 fits well the experimental points. The obtained R squared is equal to 0.999. This coefficient is a measure of how well experimental points are replicated by the mathematic model, taking into account proportion of total variation of experimental data by the model [13, 14].
Taking into account the results of the XPS as well as SEM analysis, as well as the shape of the kinetics curves the following two-step reaction scheme was proposed:
The first step can be related to the adsorption–desorption process and is described with a pseudo-first order reversible reaction. It was followed by the pseudo-first order transformation of Pd(II) chloride complex into PdCl2 species. The above reaction can be simplified to the following form assuming that the concentration of activated carbon is constant since carbon is insoluble in water:
Here k 1,obs , k 2,obs and k 3,obs are observed rate coefficients expressed as:
The sequence to the first order reactions resulting from simplified reaction, can be put forward in the form of the following differential equations system:
The general solution of the equations is correlated with Eq. 2 [15], where:
and
W 1 , W 2 , γ 1 and γ 2 were determined using the TableCurve software by fitting Eq. 2 to the experimental data, while k 1,obs , k 2,obs and k 3,obs were calculated using Mathcad software.
The pre-assembled mathematical model required validation. Therefore, further analysis was performed to observe the influence of Pd(II) initial concentration, temperature, as well as AC initial concentration on the process rate.
The influence of Pd(II) initial concentration
At first, the order of the reaction with respect to the initial concertation of Pd(II) complex ions was determined using the initial rate method. For the proposed reaction scheme, the initial rate can be given by the following formula:
Under isolation conditions, Eq. 12 can be rearranged and given in the following form:
The value of r0, can be determined as the slope of concentration vs. time plot (see Fig. 4).
To determine parameter α in Eq. 13, the logarithmic form of this equation was used (see Eq. 14).
The obtained results are shown in Fig. 5a. The order of the reaction was found to be 1.01 ± 0.07. It confirms that the assumption of first order reaction of the first step (see Eq. 7) is correct.
Moreover, from the intercept of fitted Eq. 14, it is possible to determine the k1,obs. For the studied case, it is equal to 0.081 ± 0.049. This value is in good agreement with the average value of k1,obs, which is equal to 0.097. It has to be underlined that values of kx,obs shown in Fig. 5b were obtained from the solution of assumed mathematical model. At this stage, it can be stated that assumed model in an excellent way describes the process. However, additional experiments were performed to confirm this statement.
The influence of AC initial concentration on observed process rate
In the next step, the influence of AC initial concentration on observed rate coefficients was analyzed. For this purpose, mathematically determined kx,obs values from experimentally derived kinetic curves were used. The obtained results are shown in Fig. 6.
According to Eqs. 4–6, kx,obs should be linearly dependent on concentration of AC. This assumption seems to be valid for k1,obs and k2,obs, but k3,obs is independent from the AC initial concentration. Therefore, the value of k3,obs seems to be directly equal to k3.
The influence of temperature on the process rate
One of the most important parameter in kinetic research is the activation energy. For its determination, the Arrhenius dependence was applied:
Here A x is pre-exponential factor of the xth reaction, E a,x is the activation energy of the xth reaction, T is the temperature, R is the gas constant.
To determine the E a,x and A x from the experimental data, the logarithmic form of Eq. 15 can be used:
Taking \(\ln \left( {k_{x,obs} } \right)\) v.s. 1/T plot, from its slope and intercept required parameters can be determined. The obtained results are shown in Fig. 7.
The obtained values are gathered in Table 1.
It can be seen that the activation energy for the adsorption process is two times lower as compared with the desorption step. This suggests that at low temperature, activated carbon will be able to adsorb more Pd(II) than at higher temperature, since the ratio k1,obs to k2,obs can be assumed as an equilibrium constant.
The influence of mixing rate on observed rate coefficient
The influence of the mixing rate on the observed rate coefficients was investigated at constant temperature, constant initial amount of AC and constant initial concentration of Pd(II) complex ions.
It is well known that the rate constant in the case of diffusion controlled reaction is represented by the mass-transfer coefficient k c defined as follows:
Here k c is the mass-transfer coefficient, D Pd(II) is the diffusion coefficient of Pd(II) species, δ is the thickness of the diffusion layer. The thickness of the diffusion layer on the solid phase surface depends on the fluid velocity V (for laminar flow), the kinematic viscosity of the solution µ, and the solution density ρ. Thus the rate constant is a linear function of V 1/2 [16]:
Here R AC is the radius of the AC particles.
In the studied case, fluid movement was affected by the glass stirrer dipped into the solution. The relation between mixing rate and the fluid velocity can be given as follows:
Here Mr is the mixing rate, rpm; and r is the radius of the reactor.
Since, the particles of AC are distributed in the whole volume of the reactor, the “r” term should be treated as an average radius of particles moving on circle trajectory. Taking into account the above mentioned arguments, Eq. 18 can be transformed to the following form:
Linear changes in the reaction rate constant with the square root of the mixing rate were observed for k1,obs and for k2,obs (Fig. 8). It indicates that a diffusion limited process occurs in the system.
Moreover, it can be observed that there is no influence of the mixing rate on k3,obs. This observation is in good agreement with the assumed model since k3,obs describes the rate of the process already taking place on the surface of AC and the diffusion process in not involved.
Adsorption isotherm
Experiment related with adsorption isotherm determination was performed at room temperature (c.a. 294, K) using six tightly twisted jars filled with 0.3 L of the solution, containing six different initial concentrations of Pd(II). The initial concentrations of Pd(II) were equal to 5 × 10−5, 1 × 10−4, 2.5 × 10−4, 5 × 10−4, 1 × 10−3 and 2.5 × 10−3 M.
In the studied case, the Freundlich isotherm [17] was used to describe the adsorption isotherm. The Freundlich equation can be expressed by the following way:
Here x can be calculated using Eq. 22:
Here cr is the equilibrium concentration of Pd(II) chloride ions, c0 is the initial concentration of adsorbed substance, \(\frac{x}{m}\) is the amount of Pd(II) chloride ions adsorbed on the adsorbent, m is the mass of the adsorbent (AC), β, p are constants dependent on temperature as well as the adsorbed component.
It is possible to determine β as well as the p parameters from the graph \(\log \left( {\frac{x}{m}} \right)\) v.s. \(\log \left( {c_{r} } \right)\).
In Fig. 9, only four points from six initial concentrations are shown. It is caused by the fact that equilibrium concentration for those two samples with the lowest initial concentration of 5 × 10−5, 1 × 10−4 M was lower than the detection limits of the spectrophotometer. From the linear fit, β as well as the 1/p parameters were determined and are equal to 0.36 ± 0.02 and −1.28 ± 0.12, respectively.
It is demonstrated that the Freundlich isotherm describe the process well. An attempt to fit the Langmuir isotherm to the experimental data was not successful.
From the obtained results, the adsorption capacity was calculated and it may reach a level of 371 mg/g Pd. The obtained value is high, and is much higher than that obtained for some commercially available ion exchangers [18, 19].
Equilibrium in the studied system
It was assumed that the studied process consists of two steps. In the first one, a pre-equilibrium between ionic and adsorbed species is established. The second one is related to an irreversible reaction. Under steady state conditions, it should be possible to determine the equilibrium constant for the first stage of reaction according to the following relation:
Then, the Gibbs free energy change ∆G0 for this stage may be also found as follows:
After rearrangement, Eq. 25 takes the following form:
The obtained result is shown in Fig. 10.
By fitting Eq. 26 to the experimental data, the values of ∆S0 and ∆H0 were obtained, and they are equal to −1.1 ± 0.3 J mol−1 K−1 and 0.42 ± 0.1 kJ mol−1. The positive value of ∆H0 indicates that the first step of the process is endothermic. However, the negative value of ∆S0 is unexpected. In the literature, such a phenomenon is explained in two different ways. On the one hand, it is suggested that the obtained product is more ordered than the substrate. On the other hand, this suggests that the number of degrees of freedom of adsorbed molecule is lower. Those degrees of freedom are related to the rotation and translations of species [20, 21]. In the case of adsorption processes it seems that the second explanation is more probable.
Conclusions
It was shown that the proposed kinetic model describes well the adsorption process of palladium(II) ions at the surface of activated carbon Norit Rox 0.8, and this AC exhibits high adsorption capacity. The obtained value 371 mg/g is comparable to adsorption capacity of Purolite A830 (356.88 mg/L, [22]) and is much higher than that obtained with Lewatit MonoPlus SR-7 (197 mg/g, [23]).
It is suggested that using activated carbon Norit ROX 0.8 it is possible to recover Pd(II) ions from diluted aqua solutions.
Moreover, the activation energy of each step was determined and is equal to 23.5 ± 1.3, 52.7 ± 5.5 and 22.8 ± 9.5 for the adsorption, desorption and transformation process. The lower value of activation energy for adsorption process in comparison with desorption process suggests that an increase of temperature will results in a decrease of equilibrium constant for the first stage of reaction. This might also affect observed adsorption capacity. However, this last statement has no experimental evidence and is only a hypothesis.
Kinetic studies showed that the process is controlled by diffusion. Experimental data have demonstrated that an increase of mixing rate effectively increases adsorption rate. This in turn suggests that in the case of industrial application high flow rates are required to decrease diffusion effect.
As it is known, there is no consistent model that allows the adsorption isotherm determination based on kinetic studies. Typically, to determine the adsorption isotherm, several days or weeks are required to obtain an equilibrium. Then, it is possible to determine the adsorption isotherm. Thanks to kinetic measurements, it should be possible to extrapolate, the results to determine the equilibrium state without time consuming experiments. Efforts to determine new adsorption isotherm which might be connected with kinetic data will be taken in the future.
References
Fleming CA, Nicol MJ (1984) The absorption of gold cyanide onto activated carbon. Ill. Factors influencing the rate of loading and the equilibrium capacity. J S Afr Inst Min Metall 84(4):85–93
Salarirad MM, Behnamfard A (2011) Fouling effect of different flotation and dewatering reagents on activated carbon and sorption kinetics of gold. Hydrometallurgy 109(1–2):23–28
Laxen PA (1984) Carbon-in-pulp processes in South Africa. Hydrometallurgy 13(2):169–192
Vegter NM, Sandenbergh RF, Botha AJ (1997) Concentration profiles of gold inside activated carbon during adsorption. J S Afr Inst Min Metall 97(7):299–312
Wojnicki M, Pacławski K, Socha RP, Fitzner K (2013) Adsorption and reduction of platinum(IV) chloride complex ions on activated carbon. Trans Nonferrous Met Soc China 23:1147–1156
Aktas S, Morcali MH (2011) Platinum recovery from dilute platinum solutions using activated carbon. Trans Nonferrous Met Soc China 21:2554–2558
Chen S, Xu R, Huang H, Yi F, Zhou X, Zeng H (2007) Reduction–adsorption behavior of platinum ions on activated carbon fibers. J Mater Sci 42:9572–9581
Simanova SA, Shukarev AV, Lysenko AA, Grebennikov SF, Astashkina OV (2008) Adsorption of palladium, platinum, and gold chloride complexes by carbon fibers with various structures. Fibre Chem 40(4):365–375
Wojnicki M, Pacławski K, Rudnik E, Fitzner K (2011) Kinetics of palladium(II) chloride complex reduction in aqueous solutions using dimethylamineborane. Hydrometallurgy 110(1–4):56–61
Podborska A, Wojnicki M (2017) Spectroscopic and theoretical analysis of Pd2+ –Cl–H2O system. J Mol Struct 1128:117–122. doi:10.1016/j.molstruc.2016.08.039
Wojnicki M, Luty-Błocho M, Socha R, Mech K, Pędzich Z, Fitzner K, Rudnik E (2015) Kinetic studies of sorption and reduction of gold(III) chloride complex ions on activated carbon Norit ROX 0.8. J Ind Eng Chem 29:289–297. doi:10.1016/j.jiec.2015.03.036
Wojnicki M, Fitzner K, Luty-Błocho M (2016) Kinetic studies of nucleation and growth of palladium nanoparticles. J Colloid Interface Sci 465:190–199. doi:10.1016/j.jcis.2015.11.066
Glantz SA, Slinker BK (1990) Primer of applied regression and analysis of variance. McGraw-Hill, New York
Draper NR, Smith H (2014) Applied regression analysis. Wiley, New York
Korobov V, Ochkov V (2011) Chemical kinetics with mathcad and maple. Springer, New York
Davis ME, Davis RJ (2003) Fundamentals of chemical reaction engineering. McGraw-Hill Higher Education, New York
Freundlich H (1906) Über die adsorption in Lösungen. Z Phys Chem 57:385–470
Wołowicz A, Hubicki Z (2009) Palladium(II) complexes adsorption from the chloride solutions with macrocomponent addition using strongly basic anion exchange resins, type 1. Hydrometallurgy 98:206–212
Wołowicz A, Hubicki Z (2011) Investigation of macroporous weakly basic anion exchangers applicability in palladium(II) removal from acidic solutions—batch and column studies. Chem Eng J 174(2–3):510–521. doi:10.1016/j.cej.2011.08.075
Wojnicki M, Fitzner K, Luty-BŁocho M (2015) Kinetic studies of gold recovery from dilute aqueous solutions using Fe2 + chloride ions. Trans Nonferrous Met Soc China 25(6):2027–2036. doi:10.1016/S1003-6326(15)63812-2
Stavila V, Volponi J, Katzenmeyer AM, Dixon MC, Allendorf MD (2012) Kinetics and mechanism of metal–organic framework thin film growth: systematic investigation of HKUST-1 deposition on QCM electrodes. Chem Sci 3:1531–1540. doi:10.1039/c2sc20065a
Wołowicz A, Hubicki Z (2012) Applicability of new acrylic, weakly basic anion exchanger purolite A-830 of very high capacity in removal of palladium(II) chloro-complexes. Ind Eng Chem Res 51(21):7223–7230. doi:10.1021/ie202859n
Wołowicz A, Hubicki Z (2012) Ion exchange recovery of palladium(II) from acidic solutions using monodisperse lewatitSR-7. Ind Eng Chem Res 51(51):16688–16696. doi:10.1021/ie302304c
Acknowledgements
I would like to thanks prof Krzysztof Fitzner for his constant encouragement and help in my work. I would like to thanks to Robert P. Socha, for his help during XPS measurement and data analysis. Moreover, I am grateful to Mrs. Ewa Zalecka (Brenntag Polska) and Mr. Martin Fisher (Norit Nederland BV) for kind supply of the activated carbon used in this study.
Financial support
This work was supported by the Polish Ministry of Education, with grant registered at AGH University of Science and Technology under nr 11.11.180.726/2017.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wojnicki, M. Kinetic studies of the sorption process of Pd(II) chloride complex ions from diluted aqua solutions using commercially available activated carbon. Reac Kinet Mech Cat 122, 177–192 (2017). https://doi.org/10.1007/s11144-017-1233-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-017-1233-8