Abstract
A series of potassium or cesium doped Zn0.4Co2.6O4|Al2O3 catalysts with different alkali loadings were prepared, characterized with respect to chemical composition (XRF), structure (XRD, RS) morphology (TEM), and the alkali promoter thermal stability. A strong beneficial effect on the deN2O activity of the Zn0.4Co2.6O4|Al2O3 catalyst (decrease in the T 50% by about 80 °C) was observed for both promoters at different surface coverages. It was found that in comparison to a rather narrow range of optimal cesium loading (0.5–2 atoms/nm2) a comparable promotional effect of potassium doping was observed for a slightly wider surface concentrations (0.5–3 atoms/nm2). Such difference was attributed to surface dispersion of potassium over the alumina support and the spinel active phase, while cesium was found to be located mainly on the spinel phase. For practical applications, the superiority of potassium over cesium consist in fact that a similar beneficial effect is associated with much higher thermal stability in the temperature range of the catalyst deN2O operation and lower price of the promoter precursor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrous oxide is commonly recognized as an important greenhouse gas, emitted from natural (nitrogen cycles) and anthropogenic (mainly nitric and adipic acid production) sources. The N2O emission represents 6% of the total greenhouse gas emission. However, its global warming potential (GWP) is 310 and 21 times higher than for CO2 and CH4, respectively [1]. Moreover, the presence of this gas in the atmosphere leads to destruction of the ozone layer [2]. Thus, decomposition of N2O into N2 and O2 has developed recently into a topic of a vital interest for environmental catalytic chemistry.
Some of the most efficient catalysts for N2O abatement are based on cobalt spinel [3, 4]. The catalytic performance of Co3O4 can be modified by bulk doping with transition metal cations (such as Zn, Ni, Cu, Mn, Fe) [5–10] and surface doping with alkali dopants (Na, K, Cs) [3, 8, 11–13]. Addition of alkali results in a substantial lowering of the reaction temperature window, making such catalytic system potentially suitable for the low temperature deN2O process [11]. The beneficial effect of alkali promoters has been reported to be mainly of an electronic origin [14, 15]. The presence of alkali promoters on the catalyst surface lowers the work function of the cobalt spinel facilitating redox processes that occur between the catalyst surface and the N2O reactant. It also stimulates recombination of surface oxygen intermediates, closing the catalytic cycle [11, 12]. The positive role of the alkali promoters in enhancement of the Co3O4 activity increases in the order: Na < K < Cs, and exhibits a strongly non-monotonous character as a function of surface coverage [11, 12, 16]. Among them, potassium and cesium are the most often investigated due to their strongest promotional effects. Beside the positive role in the activity enhancement the thermal stability of the alkali promoters play an important role for a long time-on-stream operation of the catalyst. The nature of the counter anion also plays an important role in the promotion of the catalytic activity of N2O decomposition. In the particular case of potassium, it has been found that the promotional effect strongly depends on the precursor nature (K2CO3, KNO3, CH3COOK, KOH), and the highest activity was observed for K2CO3. It has been assigned to the best surface dispersion and thermal stability of the potassium promoter [16].
It has been found previously by us that the synergetic effect of double promotion of cobalt spinel by Zn (bulk) and K (surface) led to spectacular decrease in the temperature of 50% N2O conversion by 200 °C [17]. However, despite such promising performance in the N2O decomposition reaction, the development of an industrial scale catalyst based on Co3O4 as a bulk active phase was hindered by the unsatisfactory mechanical stability of the catalyst extrudates and by the high price of cobalt as well. These problems can be solved quite readily by the dispersion of cobalt spinel active phase on a shaped support such as alumina, silica, magnesia or ceria [4, 18–22]. However, since the alkali promoter loading is crucial for the deN2O performance of the catalyst, and both the under- and the over-doping lead to pronounced deterioration of the activity, the alkali promotion level must be carefully adjusted for a given catalyst. This problem is becoming more subtle in the case of supported catalysts, since the promoter may be dispersed not only on the active phase but also on the carrier surface [8]. To resolve this problem, a series of potassium and cesium doped supported Zn0.4Co2.6O4|Al2O3 catalysts was prepared and tested in the N2O decomposition. The optimal range of surface K and Cs loadings and their thermal stability were determined and discussed in terms of the potential applicability in a large scale preparation practice.
Experimental
Catalyst preparation
The alpha-alumina support, prepared from commercial powder pseudoboehmite (Sasol Versal) by calcination at 1400 °C for 4 h, was supplied by the Institute of New Chemical Syntheses (INS Puławy, Poland). The supported catalyst with 15% loading of the active phase Zn0.4Co2.6O4 was prepared by the incipient wetness impregnation of the α-alumina support with the use of a 30 vol% glycerol-aqueous solution of 4.49 M Co(NO3)2 (AKTYN, pure p.a.) and 0.69 M Zn(NO3)2 (Dor-chem, pure p.a.) [23]. The obtained sample was dried at 120 °C for 1 h, and then calcined at 500 °C for 4 h. The catalyst was next doped with alkali (K or Cs) by incipient wetness impregnation with an aqueous solution of K2CO3 (POCH, pure p.a.) or Cs2CO3 (Aldrich) with concentrations corresponding to the surface loadings of potassium and cesium in the range of 0–10 atoms K/nm2 and 0–6 atoms Cs/nm2 [4, 8, 11, 12]. Then, the cycle of drying and calcination was repeated. The samples were labeled according to the content and kind of the alkali dopant (Table 1) [12].
Catalysts characterization
The BET surface area of undoped catalysts was determined by nitrogen adsorption at −196 °C in an automatic volumetric system (Autosorb-6, Quantachrome) and was equal to 8 m2 g−1.
The loading of the alkali promoter was determined using an energy-dispersive XRF spectrometer (Thermo Scientific, ARL QUANT’X) equipped with Rh anode (which generates X-rays in the range of 4–50 kV with 1 kV step and a 1 mm beam size), and with a 3.5 mm Si(Li) drifted crystal detector operated with a Peltier cooling. For quantitative analysis, a series of metallic calibration standards and the UniQuant software were used.
X-ray diffraction measurements were carried out by Rigaku MiniFlex diffractometer with Cu Kα radiation at 10 mA and 10 kV. The diffractograms were recorded in a 2θ range from 10° to 90° with the 0.02° step, and the counting time of 1 s per step. The diffractograms were indexed using the ICSD database.
The phase composition and structure verification were examined by Raman spectroscopy using a Renishaw InVia spectrometer with a confocal Leica DMLM microscope and a CCD detector with the excitation wavelength of 785 nm. The Raman scattered light was collected in the spectral range of 100–900 cm−1 with a resolution of 1 cm−1. At least five scans were accumulated to ensure a sufficient signal to noise ratio.
TEM observations were carried out by means of a FEI Tecnai Osiris microscope with an X-FEG Schottky field emitter operated at 200 kV. The instrument was also equipped with a Super-X EDX (energy dispersive X-ray) windowless detector system with a 4-sector silicon drift detector (SDD). The Z-contrast images were acquired using a high angle annular dark field (HAADF) detector in the scanning mode. HAADF-STEM images coupled with EDX elemental mapping were acquired using Bruker Esprit software for the sample drift correction. For the microscopic measurements the samples were deposited on a lacey carbon-coated copper grid.
The stability of potassium and cesium was investigated by the species resolved – thermal alkali desorption (SR–TAD) method [24]. The experiments were carried out in a vacuum apparatus with a background pressure of 10−8 mbar. The samples, in the form of wafers of 13 mm in diameter with a mass of about 100 mg, were heated from 25 to 600 °C. The desorption flux of alkali atoms, S/pA, was determined by means of a surface ionization detector. During the measurements, the samples were biased with a positive potential +5 V to suppress thermionic emission of electrons. The resultant positive current was measured directly with a digital electrometer (Keithley 6512), and averaged over four independent data points for each temperature. The activation energy (E des) for alkali desorption was calculated with an assumption that the process follows first order kinetics by applying Arrhenius equation justified elsewhere [25].
Catalytic tests
Catalytic tests of nitrous oxide decomposition were carried out for all catalysts in a temperature programmed surface reaction (TPSR) mode. A 300 mg sieve fraction of the catalyst (0.2–0.3 mm) was placed in a quartz flow reactor, with a thermocouple positioned just above the catalyst bed. The reaction was performed in the temperature range of 20–600 °C (with a heating rate of 10°/min) and at the atmospheric pressure, using a gas mixture of 5% N2O in He with the total gas flow of 30 ml/min (GHSV = 7000 h−1; calculated for catalyst volume). Before measuring the catalysts activity, the samples were pretreated in situ by heating up to 600 °C. Changes in the gas composition were monitored by a quadrupole mass spectrometer (RGA200, SRS, lines m/z = 44 (N2O), 32 (O2), 30 (NO), 28 (N2), 18 (H2O)).
Results and discussion
The synthesized catalysts were examined with respect to the spinel active phase formation (XDR, Raman spectroscopy), spinel and alkali loading (XRF), their morphology including alkali distribution mapping (TEM/EDX) and thermal stability of the alkali promoters (SR-TAD). The XRF analysis of the investigated samples indicated the presence of 17.7 wt% of the spinel Zn0.4Co2.6O4 phase, close to the intended loading of 15 wt%. The alkali content, which was the main optimization parameter in this study, is summarized in Table 1.
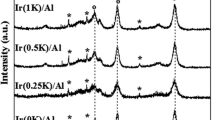
The crystalline structure of the active phase of the supported catalyst was examined by XRD and Raman techniques. A typical XRD pattern of the 2K-Zn0.4Co2.6O4|Al2O3 catalyst is shown in Fig. 1, as an example. Apart from the dominant diffraction peaks due to the α-Al2O3 support, several weaker diffraction lines at 2Θ = 31.3°; 36.9°; 44.9°; 55.7°; 59.5°; 65.4°, corresponding to the (220), (311), (400), (422), (511) and (440) reflection planes of the cobalt spinel phase, are clearly visible [8]. No apparent changes in the XRD patterns were observed upon alkali doping of the samples, indicating that the spinel active phase remains essentially intact upon the alkali promotion.
Raman spectra of the investigated catalysts are shown in Fig. 2. Five bands located at 195 (F2g), 487 (Eg), 526, 624 (F2g) and 694 (A1g) cm−1 confirmed the presence of the spinel phase, and absence of any impurities [26]. The apparent asymmetry of the Raman A1g and F2g peaks is a result of substitution of the zinc for cobalt in the Co3O4 framework, implying formation of the intended Zn x Co(1−x)Co2O4 solid solution [17]. As a result, analysis of the XRD and RS data eliminates a possibility of undesired ZnO segregation from the spinel active phase. In the case of alkali-doped catalysts there is no difference observed on Raman spectra in comparison with the un-doped samples as it was previously observed [8].
STEM-HAADF images of the alkali promoted catalysts show the dispersion of the spinel nanocrystals over the alumina micrograins (Figs. 3a1 and 3b1). The Al2O3 grains of a rounded shape and ~0.5–2 µm in size are decorated by the Zn0.4Co2.6O4 spinel nanocrystals. A detailed inspection of the catalyst nanostructure of the potassium/cesium-promoted Zn0.4Co2.6O4|α-Al2O3 catalysts showed that the typical spinel crystallites exhibit polyhedral shape (faceted) with the dominant (100) and (111) terminations as revealed by the image analysis and inverse Wulff construction [23]. The spinel active phase is fairly well dispersed over the alumina carrier, and the average size of the Zn0.4Co2.6O4 crystallites is distinctly smaller for the potassium-doped samples (10–20 nm) in comparison to the cesium-promoted catalyst (40–50 nm). The spinels nanograins are both directly attached to the alumina support surface or connected to another nanograins by forming small aggregates (Figs. 3a1 and 3b1).
STEM-HAADF images for: a 1 K-Zn0.4Co2.6O4|Al2O3 calcined at 500 °C, b 1 Cs-Zn0.4Co2.6O4|Al2O3 calcined at 500 °C. HAADF Z-contrast images showing the size and distribution of the spinel nanocrystals over larger grains of Al2O3 support. The corresponding EDX spectra (a 5 and b 5 ) along with the associated EDX chemical maps (a 2 –a 4 and b 2 –b 4 ) showing element repartition between the Al2O3 support and Zn0.4Co2.6O4 spinel active phase
The presence of the alkali promoter (potassium or cesium) on the Zn0.4Co2.6O4|Al2O3 catalyst was confirmed by the STEM-EDX measurements. Figs. 3a5 and 3b5 show the corresponding EDX spectra with the well-developed signals characteristic for potassium (K α at 3.31 eV and Kβ at 3.59 eV) and for cesium (Lα at 4.28 eV, Lβ 1 at 4.62 eV, Lβ 2 at 4.93 eV). The STEM-EDX elemental mapping revealed apparent differences in the alkali surface redistribution between the spinel and the support (Figs. 3a4 and 3b4). Whereas in the case of the K-Zn0.4Co2.6O4|Al2O3 catalyst, potassium is distributed between the spinel and occasionally the alumina carrier surface, for the cesium doped samples, the alkali promoter is mainly confined to the spinel nanograins. Such behavior can be accounted for by the results of thermal alkali desorption studies. The results for potassium and cesium desorption from the investigated catalysts are shown in Fig. 4. They clearly reveal a difference in the thermal stability between these promoters. The onset for cesium desorption is observed at ~350 °C while in the case of potassium it is shifted to ~500 °C. A clear exponential character of the desorption curve as a function of temperature implies that the desorption occurs through single barrier associated with the Cs–Osurf bond breaking. Indeed, the Arrhenius plot (shown in the insert) is linear in the investigated temperature range and the activation energy, equal to 1.3 eV, can be reliably determined. This value is characteristic of Cs atom desorption from the cobalt spinel surface at low coverages as shown and discussed in our previous paper [12]. In the case of potassium the desorption process is more complex as the Arrhenius plot reveals two desorption energies of 2.6 and 1.2 eV (at low temperature, shown in [8]). They can be associated with potassium desorption from the cobalt spinel phase and the alumina support. In conclusion, the microscopic and thermal desorption studies revealed consistently that potassium is distributed between the active phase (major part) and the support (minor part), while cesium is present mainly at the spinel phase.
The catalytic deN2O activity of the two series of the potassium and cesium doped Zn0.4Co2.6O4|Al2O3 samples of various alkali loadings was examined by means of the TPSR measurements. The results expressed as N2O conversion versus temperature are collated in Fig. 5. The comparison of the N2O conversion profiles for the K-doped (Fig. 5a) and Cs-doped (Fig. 5b) Zn0.4Co2.6O4|Al2O3 catalysts reveals strong impact of the alkali promotion on the extent of the N2O conversion. It is manifested by pronounced shifts of the conversion curves towards lower and higher temperatures upon doping. The highest activity was found for the samples with the potassium loading of 0.116 wt% (2 atoms per nm2) and cesium loading of 0.086 wt% (1 atom per nm2). It should be noted, however, that the samples with the highest surface concentration of potassium (5 K, 10 K) or cesium (4 Cs, 6 Cs) exhibit lower activity than the undoped Zn0.4Co2.6O4|Al2O3 catalyst.
The results of catalytic activity expressed as temperature of 50% of conversion are plotted as a function of potassium and cesium loadings in Fig. 6a (alkali loading expressed in wt% determined from XRF) and Fig. 6b (alkali loading expressed as a surface coverage in atoms per nm2, calculated based on total BET area). It can be inferred that for both series the doping with alkali (K or Cs) showed non-monotonous behavior with the minimum in the range of 0.5–3 atoms per nm2 for potassium and 0.5–2 atoms per nm2 for cesium. It is worth to mention, that for both alkali promoters the T 50% can be lowered down to ~350 °C, shifting down thereby the activity window by ~80 °C.
The beneficial effect of alkali promotion of bulk cobalt spinel was previously studied by work function measurements and DFT calculations [3]. Those studies allowed us to explain this effect in terms of electronic promotion gauged by a lowering of the catalyst work function and facilitation of the electron transfer from the catalyst surface to the reactant molecule, and thus N–O bond dissociation. As discussed therein, the alkali metals of low ionization potential (IP Cs = 3.89 eV; IP K = 4.34 eV) strongly modify the surface electronic properties of the catalyst. At low surface loadings, the deposited alkali form diffuse surface dipoles (Csδ+–O δ− surf or Kδ+–O δ− surf ) due to electron density donation to the spinel catalyst. Such dipoles counterbalance the surface electrostatic potential, facilitating electron shuttling between the catalyst and the N2O reactant (N2O + e −(Co) → N2 + O −surf ) in the reduction step and the O− intermediates (2O −surf → O2(g) + 2e −(Co) ) in the oxidation step, that closes the catalytic cycle. The redox centers are constituted by the spinel octahedral cobalt ions [27]. However, when alkali surface coverage increases (alkali dipoles come closer) the beneficial effect of surface dipoles is ceasing due to the depolarization effect [28]. As a consequence, after passing the optimal loading, the activity falls down with the increasing alkali coverage (Fig. 6). It should be also noted that the negative effect at excessive coverages due to the large size of alkali cations may also results from the cobalt active sites blocking.
A comparison of the optimal level of alkali-doping for the supported spinel catalysts (0.5–2 for Cs and 0.5–3 for K) shows that they appreciably differ. This can be interpreted in terms of different surface stability of these both promoters and their surface dispersion (see microscopic observations). Although the activity enhancement is similar for these both promoters from practical point of view the superiority of potassium seems to be evident due to a considerably broader range of optimal loading and distinctly lower price of the promoter precursor. Thus, potassium addition should be much more easily to handle while upscaling the catalyst preparation for industrial applications. Another important point, speaking in favor of potassium, is its significantly higher thermal stability (the difference in the desorption onset between K and Cs reaches 150 °C, Fig. 4) in the temperature range of the catalyst practical operation for N2O removal from the tail gases of nitric acid plants (350–450 °C).
Conclusions
A strong beneficial effect of potassium or cesium on the deN2O activity of the Zn0.4Co2.6O4|Al2O3 catalyst, gauged by the decrease in the T 50% by about 80 °C, was observed for both promoters at different optimal surface coverages (0.5–3 K atoms/nm2 and 0.5–2 Cs atoms/nm2). This founding was accounted for by a different surface stability of these both promoters and their dispersion on the catalyst surface. From a practical point of view, the superiority of potassium over cesium results from a broader range of the optimal loading and the much higher thermal stability in the temperature range of the catalyst practical operation for N2O removal from the tail gases of nitric acid plants.
References
Pérez-Ramírez J, Kapteijn F, Schöffel K, Moulijin JA (2003) Formation and control of N2O in nitric acid production: where do we stand today? Appl Catal B 44:117–151
Trogler WC (1999) Physical properties and mechanisms of formation of nitrous oxide. Coord Chem Rev 187:303–327
Zasada F, Stelmachowski P, Maniak G et al (2009) Potassium promotion of cobalt spinel catalyst for N2O decomposition-accounted by work function measurements and DFT modelling. Catal Lett 127:126–131
Grzybek G, Stelmachowski P, Gudyka S et al (2016) Strong dispersion effect of cobalt spinel active phase spread over ceria for catalytic N2O decomposition: the role of the interface periphery. Appl Catal B 180:622–629
Yan L, Ren T, Wang X et al (2003) Excellent catalytic performance of ZnxCo1−xCo2O4 spinel catalysts for the decomposition of nitrous oxide. Catal Commun 4:505–509
Yan L, Ren T, Wang X et al (2003) Catalytic decomposition of N2O over MxCo1−xCo2O4 (M = Ni, Mg) spinel oxides. Appl Catal B 45:85–90
Abu-Zied BM, Soliman SA, Abdellah SE (2015) Enhanced direct N2O decomposition over CuxCo1−xCo2O4 (0.0 ≤ x≤1.0) spinel-oxide catalysts. J Ind Eng Chem 21:814–821
Grzybek G, Wójcik S, Legutko P et al (2017) Thermal stability and repartition of potassium promoter between the support and active phase in the K-Co2.6Zn0.4O4|α-Al2O3 catalyst for N2O decomposition: Crucial role of activation temperature on catalytic performance. Appl Catal B 205:597–604
Karásková K, Obalová L, Kovanda F (2011) N2O catalytic decomposition and temperature programmed desorption tests on alkali metals promoted Co-Mn-Al mixed oxide. Catal Today 176:208–211
Konsolakis M (2015) Recent advances on nitrous oxide (N2O) decomposition over non-noble-metal oxide catalysts: catalytic performance, mechanistic considerations, and surface Chemistry aspects. ACS Catal 5:6397–6421
Stelmachowski P, Maniak G, Kotarba A, Sojka Z (2009) Strong electronic promotion of Co3O4 towards N2O decomposition by surface alkali dopants. Catal Commun 10:1062–1065
Grzybek G, Stelmachowski P, Gudyka S et al (2015) Insights into the twofold role of Cs doping on deN2O activity of cobalt spinel catalyst-towards rational optimization of the precursor and loading. Appl Catal B 168–169:509–514
Asano K, Ohnishi C, Iwamoto S et al (2008) Potassium-doped Co3O4 catalyst for direct decomposition of N2O. Appl Catal B 78:242–249
Ohnishi C, Asano K, Iwamoto S et al (2007) Alkali-doped Co3O4 catalysts for direct decomposition of N2O in the presence of oxygen. Catal Today 120:145–150
Pasha N, Lingaiah N, Babu NS et al (2008) Studies on cesium doped cobalt oxide catalysts for direct N2O decomposition in the presence of oxygen and steam. Catal Commun 10:132–136
Maniak G, Stelmachowski P, Kotarba A et al (2013) Rationales for the selection of the best precursor for potassium doping of cobalt spinel based deN2O catalyst. Appl Catal B 136–137:302–307
Inger M, Wilk M, Saramok M et al (2014) Cobalt spinel catalyst for N2O abatement in the pilot plant operation-long-term activity and stability in tail gases. Ind Eng Chem Res 53:10335–10342
Boissel V, Tahir S, Koh CA (2006) Catalytic decomposition of N2O over monolithic supported noble metal-transition metal oxides. Appl Catal B 64:234–242
Shen Q, Li L, Li J et al (2009) A study on N2O catalytic decomposition over Co/MgO catalysts. J Hazard Mater 163:1332–1337
Christoforou SC, Efthimiadis EA, Vasalos IA (2002) Catalytic conversion of N2O to N2 over metal-based catalysts in the presence of hydrocarbons and oxygen. Catal Lett 79:137–147
Bueno-López A, Such-Basáñez I, de Lecea CSM (2006) Stabilization of active Rh2O3 species for catalytic decomposition of N2O on La-, Pr-doped CeO2. J Catal 244:102–112
Reddy PSS, Seshu Babu N, Pasha N et al (2008) Influence of microwave irradiation on catalytic decomposition of nitrous oxide over Rh/Al2O3 catalyst. Catal Commun 9:2303–2307
Gudyka S, Grzybek G, Gryboś J et al (2017) Enhancing the deN2O activity of the supported Co3O4|α-Al2O3 catalyst by glycerol-assisted shape engineering of the active phase at the nanoscale. Appl Catal B 201:339–347
Kotarba A, Rozek W, Serafin I, Sojka Z (2007) Reverse effect of doping on stability of principal components of styrene catalyst: KFeO2 and K2Fe22O34. J Catal 247:238–244
Kotarba A, Dmytrzyk J, Raróg-Pilecka W, Kowalczyk Z (2003) Surface heterogeneity and ionization of Cs promoter in carbon-based ruthenium catalyst for ammonia synthesis. Appl Surf Sci 207:327–333
Hadjiev VG, Iliev MN, Vergilov IV (1988) The Raman spectra of Co3O4. J Phys C 21:L199–L201
Stelmachowski P, Maniak G, Kaczmarczyk J et al (2014) Mg and Al substituted cobalt spinels as catalysts for low temperature deN2O-Evidence for octahedral cobalt active sites. Appl Catal B 146:105–111
Somorjai GA (1993) Introduction to surface chemistry and catalysis. Wiley, New York
Acknowledgements
The authors would like to acknowledge the Polish National Centre for Research and Development funding awarded by the decision number PBS2/A5/38/2013. The research was partially carried out with the equipment purchased thanks to the financial support of the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (Contract No. POIG.02.01.00-12-023/08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ciura, K., Grzybek, G., Wójcik, S. et al. Optimization of cesium and potassium promoter loading in alkali-doped Zn0.4Co2.6O4|Al2O3 catalysts for N2O abatement. Reac Kinet Mech Cat 121, 645–655 (2017). https://doi.org/10.1007/s11144-017-1188-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-017-1188-9