Abstract
Mechanochemical synthesis has been successfully employed to prepare Cu0–Al2O3 composite powders. The formation of the designed compound is due to the self-propagating reaction of CuO and Al0 powders by high-energy ball milling using a planetary ball mill (Pulverissette 6, Fritsch GmbH). The products were characterized by means of nitrogen adsorption (BET surface area), X-ray diffraction, scanning electron microscopy and temperature-programmed reduction of hydrogen. The catalytic properties of the prepared materials were tested in the methanol steam reforming (SRM) process. The results show that the Cu0–Al2O3 system is active towards hydrogen in SRM. Hydrogen yields are satisfactory; however, methanol conversion to CO as a by-product is observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copper-based catalysts are known due to their high activity and selectivity in processes such as methanol synthesis, water gas shift reaction, selective catalytic reduction of NO x , oxidative methanol steam reforming (SRM) as well as in SRM [1–8]. They are especially important in the last case in spite of the insufficient stability of the process [9–14]. Several possibilities are reported in the literature on the improvement of catalytic properties. Special attention is directed to methods for producing the catalyst considering, among other things, high dispersion on the support and small particle sizes of Cu and also the addition of promoters [14–19]. Recently, a detailed review of steam reforming catalysts, also based on copper, was presented by Sá et al. [14].
The conventional methods of syntheses include mainly wet ones, i.e. impregnation and co-precipitation from aqueous solutions or other solvents. These methods are complex, require the use of heat treatment at high temperatures and/or pressures or maintain proper pH of solution. Moreover, they generate significant amounts of environmentally harmful waste.
Various examples of catalysts can be tested using the above techniques, e.g. the Cu (Zn) (Zr)–alumina prepared through the wet impregnation of metal nitrates as well as co-precipitation using a sodium carbonate and then calcination in both cases (in air at 400 °C for 4 h) [1, 15]. The co-precipitation method of Cu/Zn/Zr/Ga oxides applied citric acid solution and then solid residue was thermally treated [20].
Liu et al. [21] had demonstrated not only the conventional, but also a soft reactive milling procedure for copper–manganese spinel synthesis. The precursors of Cu and Mn were copper hydroxocarbonate and manganese carbonate, respectively, together with solid oxalic acid. The results show the superior performance of mechanochemically synthesized Cu–Mn spinel catalyst in the SRM for hydrogen production as compared with the material obtained by co-precipitation.
Taking the environmental problems into account, mechanochemical syntheses are attracting due to their simplicity, freedom of waste and energy efficiency. These syntheses enable the production of new functional materials (including catalysts) with improved and/or novel physical and chemical properties [22–24].
The quality of products strongly depends on the reagents’ nature as well as on the mechanochemical treatment conditions. In the CuO–Al system, it is possible to synthesize different kinds of composite powders, e.g. Cu(Al)–Al2O3, Cu9Al4–Al2O3, CuAl2–Al2O3 [25, 26].
The aim of this study was the formation of well-dispersed Cu0 on Al2O3 via a self-propagating reaction in the CuO–Al0 system realized by high-energy ball milling and to show the catalytic characteristics of the copper–alumina composite in the steam reforming of methanol.
Experimental
Mechanochemical synthesis of the Cu0–Al2O3 catalyst and characterization methods
Cu0–Al2O3 composite powder was synthesized by the mechanochemical treatment of CuO (p.a. Fluka) with Al0 (p.a. POCh) powder in 3:2 molar ratio. Milling was carried out in a planetary ball mill (Pulverissette 6, by Fritsch GmbH) under Ar using a vial and balls made of WC. The milling conditions were as follows: 550 rpm, BPR = 40:1, milling time ranged from 0 to 10 h.
X-ray powder diffraction (XRD) patterns were recorded on a Philips X’Pert diffractometer (CuKα) in the 2θ range of 10–90°. A Hitachi S-4700 instrument (scanning electron microscopy [SEM]) equipped with an energy dispersive X-ray spectrometer was used for microstructural examination and elemental microanalysis. The BSE imaging and EDX elemental analyses were carried out at an electron beam voltage of 20 kV. The specific surface area was estimated by the BET method, and the pore volume and pore size were measured using the BJH method (N2 adsorption/desorption isotherms at 77 K) using a Micromeritics ASAP 2020 V3.04 H apparatus, after out-gassing at 250 °C for 4 h.
Temperature-programmed reduction (H2-TPR) analyses
Temperature-programmed reduction of hydrogen (H2-TPR) is often used to obtain information about metal species with different types of dispersion, and to identify the redox property of a carrier and catalysts system. TPR profiles of copper-supported materials are usually complex, mainly due to particle size of CuO and its interaction with a carrier.
Reduction was carried out from 50 to 650 °C with a temperature heating rate of 10 °C min−1 using a mixture of hydrogen and helium (5 % vol. H2). A thermal conductivity detector (TCD) determined hydrogen consumption. Zeńczak et al. [27] described apparatus used in the present measurements.
Catalytic tests: steam reforming of methanol (SRM)
The catalytic activity of the initial CuO–Al mixture and Cu0–Al2O3 milling products were studied for comparison. The tests were performed in a pulse microreactor giving an excellent possibility to observe the dynamics of changes in catalyst activity. The microreactor was connected on-line with the chromatograph SRI 8610C equipped with TCD and FID detectors. Porapak Q and 5A molecular sieves columns were used for product separation. Argon as a carrier gas (30 ml min−1), catalyst in an amount of 0.1 g (fractions from 0.2 to 0.3 mm), and injections (0.6 μl) of CH3OH–H2O (1:1) pulses up to 40 were used. The reaction temperature was kept 300 °C.
Results and discussion
Characteristics of materials used in SRM tests
Fig. 1a–c illustrate the XRD patterns of the initial mixture of CuO with Al and milling products after 5 and 10 h. After 5 h as well as 10 h of milling, the metallic copper and amorphous Al2O3 were created (according to reaction 1) and some amount of unreacted CuO and Al reagents were also present.
SEM images of CuO–Al samples are shown in Fig. 2. It can be seen that the textural and crystal size changes as a result of mechanochemical reduction CuO to Cu. In Fig. 2a, only two phases are present, i.e. initial metallic Al grains partially covered by CuO. After 5 h of mechanochemical treatment, Cu species appeared (Fig. 2b, c).
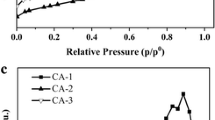
Al system are shown in Table 1. After 5 h of milling, an increase of surface area was observed. Generally, the BET values are rather low, although the milling process caused threefold increase of surface area. Elongation of milling time (for 10 h) involves the agglomeration of powder.
The H2-TPR profiles are shown in Fig. 3. The initial CuO–Al mixture shows only one reduction peak with a maximum at 315 °C (Fig. 3a). This peak can be attributed to the reduction of CuO(II) to Cu0. Fig. 3b shows the reduction of Cu0–Al2O3–(CuO + Al) system (after 5 h of milling). Lowering the reduction temperature and decreasing of the hydrogen consumption are evident because of a smaller amount of unreacted CuO as well as the presence of the activated form of copper oxide. There are two maxima at 183 and 255 °C. This was also observed by Fierro et al. [29].
Phenomena occurring in Cu–Al2O3 catalytic system during SRM process
According to reaction 2, the molar composition of main products, H2 and CO2, as well as by-product CO (reaction 3), were registered as a function of injection numbers of reactant mixture.
The total conversion of methanol for all investigated samples is shown in Fig. 4, indicating high values of conversion degrees for milling samples.
The course of hydrogen yield is presented in Fig. 5, and molar ratios of H2/CO2 are collected in Table 2. These values depend on the amount of CuO in the systems. They are lower than expected one, i.e. stoichiometric value (3.0).
Characteristics of catalysts after SRM reaction
Fig. 6 shows the XRD patterns of Cu0–Al2O3–(CuO + Al) system after catalytic tests. Higher amounts of metallic copper formed while the reduction of CuO is evident (compare with Fig. 1a–c). This is confirmed by SEM/BSE images of copper clusters shown in Fig. 7a–c.
H2-TPR measurements were also conducted on the Cu–Al2O3/5 h system after the SRM process. A very low value of H2 consumption indicates the presence of CuO traces (Fig. 3c).
Conclusions
The results showed the possibility of hydrogen production by the steam reforming of methanol using a mechanochemically treated CuO–Al system, which creates a copper-based catalyst. The metallic copper formed on Al2O3 is the active phase in the SRM process. The selectivity towards hydrogen production is satisfactory at 300 °C (70 mol%), although the methanol reforming to CO is still present.
References
Matter PH, Ozkan US (2005) J Catal 234:463–475
Turco M, Bagnasco G, Costantino U, Marmottini F, Montanari T, Ramis G, Busca G (2004) J Catal 228:43–55
Chang F-W, Kuo W-Y, Yang H-C (2005) Appl Catal A 288:53–61
Zhang XR, Shi P, Zhao J, Zhao M, Liu C (2003) Fuel Process Technol 83:183–192
Pérez-Hernández R, Mondragón Galicia G, Mendoza Anaya D, Palacios J, Angeles-Chavez C, Arenas-Alatorre J (2008) Int J Hydrog Energy 33:4569–4576
Kurr P, Kasatkin I, Girgsdies F, Trunschke A, Schlögl R, Ressler T (2008) Appl Catal A 348:153–164
Lin K-S, Pan C-Y, Chowdhury S, Lu W, Yeh C-T (2011) Thin Solid Films 519:4681–4686
Yang H-M, Chan M-K (2011) Catal Commun 12:1389–1395
Purnama H (2003) Steam reforming of methanol. PhD Thesis, TU Berlin (D 83), pp 10, 16–19
Mastalir A, Frank B, Szizybalski A, Soerijnto H, Deshpande A, Niederberger M, Schomäcker R, Schlögl R, Ressler T (2005) J Catal 230:464–475
Matsumura Y, Ishibe H (2009) Catal J 268:282–289
Kameoka S, Okada M, Sai AP (2008) Catal Lett 120:252–256
Kuznetsow VV, Vitovsky OV (2008) J Eng Thermophys 17:191–195
Sá S, Silva H, Brandão L, Sousa JM, Mendes A (2010) Appl Catal B 99:43–57
Patel S, Pant KK (2006) J Porous Mater 13:373–378
Wang L, Ding W, Liu Y, Fang W, Yang Y (2010) J Nat Gas Chem 19:487–492
Meille V (2006) Appl Catal A 315:1–17
Lin K-S, Chowdhury S, Yeh H-P, Hong W-T, Yeh C-T (2011) Catal Today 164:251–256
Mrad M, Gennequin C, Aboukaïs A, Abi-Aad E (2011) Catal Today 176:88–92
Lachowska M (2010) Reac Kinet Mech Cat 101:85–91
Liu Q, Wang L-C, Chen M, Liu Y-M, Cao Y, He H-Y, Fan K-N (2008) Catal Lett 121:144–150
Wieczorek-Ciurowa K, Gamrat K (2007) J Therm Anal Calorim 88(1):213–217
Wieczorek-Ciurowa K (2010) In: Sopicka-Lizer M (ed) Ch. 9: mechanochemical synthesis of metallic–ceramic composite powders: high-energy ball milling: mechanochemical processing of nanopowders. Woodhead Publishing Ltd., Cambridge
Wieczorek-Ciurowa K, Rakoczy J, Błońska-Tabero A, Filipek E, Nizioł J, Dulian P (2011) Catal Today 176:314–317
Wieczorek-Ciurowa K, Oleszak D, Gamrat K (2007) Chem Sustain Dev 15:255–258
Wieczorek-Ciurowa K, Oleszak D, Gamrat K (2008) Rev Adv Mater Sci 18:248–252
Zeńczak K, Michorczyk P, Rachwalik R, Ogonowski J (2010) Czasopismo Techniczne 10-Ch:353–360
Schaffer GB, McCormick PG (1990) Metall Trans A 21A:2789–2794
Fierro G, Lo Jacono M, Inversi M, Porta P, Lavecchia R, Cioci F (1994) J Catal 148:709–721
Acknowledgments
The study is financial supported by the Polish Ministry of Science and Higher Education (Project No. PB N N209 145136) and The European Union through the European Social Fund within “Cracow University of Technology Development Program—top quality teaching for the prospective Polish engineers; University of the 21st century” project (Contract No. UDA-POKL.04.01.01-00-029/10-100). Authors thank to Dr. P. Michorczyk (CUT) for access to the device for measuring H2-TPR.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Rakoczy, J., Nizioł, J., Wieczorek-Ciurowa, K. et al. Catalytic characteristics of a copper–alumina nanocomposite formed by the mechanochemical route. Reac Kinet Mech Cat 108, 81–89 (2013). https://doi.org/10.1007/s11144-012-0503-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-012-0503-8