Abstract
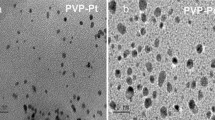
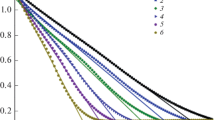
The kinetics and stoichiometry of catalytic decomposition of hydrazine in 0.01 M NaOH solutions in the presence of unstabilized (“gray” colloid) and stabilized with sodium polyacrylate (“brown” colloid) platinum nanoparticles were studied. The main decomposition products are ammonia and N2 with H2 impurity (up to 1.5%), i.e., hydrazine decomposition predominantly follows the stoichiometric equation 3N2H4 = 4NH3 + N2. The catalytic activity was studied as influenced by nanoparticle size distribution. Despite higher nanoparticle dispersion, the catalytic activity of the stabilized “brown” colloid is lower than that of the “gray” colloid. The reaction mechanism is discussed.
Similar content being viewed by others
REFERENCES
Koltunov, V.S., Kinetika reaktsii aktinoidov (Kinetics of Actinide Reactions), Moscow: Atomizdat, 1974.
Finlayson, M.V. and Mowat, J.A.S., Electrochem. Technol., 1965, vol. 3, p. 148.
Radioactive Waste Management Series. Denitration of Radioactive Liquid Waste, Cecille, L. and Halaszovich, S., Eds., London: Graham, 1986.
Krot, N.N., Shilov, V.P., Dzyubenko, V.I., et al., Radiokhimiya, 1995, vol. 37, no.1, pp. 23–27.
Ananiev, A.V., Broudic, J.-C. and Brossard, Ph., Appl. Catal. A: Gen., 2003, vol. 242, no.1, pp. 1–10.
Anan’ev, A.V. and Shilov, V.P., Radiokhimiya, 2004, vol. 46, no.4, pp. 348–355.
Lewis, L.N., Chem. Rev., 1993, vol. 93, p. 2692.
Gates, B.C., Chem. Rev., 1995, vol. 95, p. 511.
Henglein, A., Ershov, B.G., and Malow, M., J. Phys. Chem., 1995, vol. 99, p. 14129–14136.
Ershov, B.G., Izv. Ross. Akad. Nauk, Ser. Khim., 2001, no. 4, pp. 600–605.
Ershov, B.G. and Sukhov, N.L., Zh. Fiz. Khim., 2001, vol. 75, no.8, pp. 1430–1434.
Dosage spectrophotometrique de l’hydrazine. Méthodes d’analyse 1968 du Commissariat a l’Energie atomique, Paris: CETAMA, 1968, no. 241.
Moelwyn-Hughes, E.A., The Chemical Statics and Kinetics of Solutions, London: Academic, 1971. Translated under the title Ravnovesiya i kinetika reaktsii v rastvorakh, Moscow: Khimiya, 1975, p. 114.
Ershov, B.G. and Sukhov, N.L., Mendeleev Commun., (in press).
Friswell, N.J. and Govenlock, B.G., Advances in Free Radical Chemistry, Williams, G.H., Ed., London: Academic, 1967, no. 2, pp. 1–45.
Author information
Authors and Affiliations
Additional information
Translated from Radiokhimiya, Vol. 46, No. 6, 2004, pp. 531–535.
Original Russian Text Copyright © 2004 by Anan’ev, Boltoeva, Sukhov, Bykov, Ershov.
Rights and permissions
About this article
Cite this article
Anan’ev, A.V., Boltoeva, M.Y., Sukhov, N.L. et al. Catalytic decomposition of hydrazine in weakly alkaline solutions on platinum nanoparticles. Radiochemistry 46, 578–582 (2004). https://doi.org/10.1007/s11137-005-0031-8
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11137-005-0031-8