Abstract
Objective
The aim of this systematic review with meta-analysis was to evaluate the effectiveness of RMT in internal and central nervous system disorders, on pulmonary function, exercise capacity and quality of life.
Methods
The inclusion criteria were (1) publications designed as Randomized Controlled Trial (RCT), with (2) participants being adults with pulmonary dysfunction caused by an internal disease or central nervous system disorder, (3) an intervention defined as RMT (either IMT or EMT) and (4) with the assessment of exercise capacity, respiratory function and quality of life. For the methodological quality assessment of risk of bias, likewise statistical analysis and meta-analysis the RevMan version 5.3 software and the Cochrane Risk of Bias Tool were used. Two authors independently analysed the following databases for relevant research articles: PubMed, Scopus, Cochrane Library, Web of Science, and Embase.
Results
From a total of 2200 records, the systematic review includes 29 RCT with an overall sample size of 1155 patients. Results suggest that patients with internal and central nervous system disorders who underwent RMT had better quality of life and improved significantly their performance in exercise capacity and in respiratory function assessed with FVC and MIP when compared to control conditions (i.e. no intervention, sham training, placebo or conventional treatments).
Conclusion
Respiratory muscle training seems to be more effective than control conditions (i.e. no intervention, sham training, placebo or conventional treatment), in patients with pulmonary dysfunction due to internal and central nervous system disorders, for quality of life, exercise capacity and respiratory function assessed with MIP and FVC, but not with FEV1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early identification of respiratory muscle weakness is important as a diagnostic and prognostic factor as well as for the implementation of appropriate therapeutic strategies [1]. Inspiratory muscle dysfunction due to obstructive pulmonary disease or neuromuscular disorders can result in reductions in inspiratory muscle strength and endurance, thus contributing to dyspnea, decreased exercise tolerance, and impaired quality of life [2]. Impairment of respiratory function after stroke related to dysphagia and ineffective cough may increase the risk of pneumonia, which has been reported as a leading cause of extravascular death after stroke [3]. In patients with respiratory disorders, muscle weakness can lead to dyspnea and hypercapnia which affects components of physical performance [4]. Impaired respiratory muscle strength and functional capacity are often reported in patients with lung or heart disease [5]. Breathing alterations are related to a decrease in physical activity and therefore, to a reduction in the ability to carry out the activities of daily life [6]. Thus, implementing interventions that have the potential to improve respiratory function and, consequently, to prevent morbidity and mortality for a chronically ill patients is vindicated [7].
Respiratory muscle training (RMT) has long been used in many medical disciplines [8]. Respiratory muscles respond to training similarly to any other skeletal muscle [9]. In this regard, RMT consists of repetitive breathing exercises with hand-held respiratory trainer devices to provide pressure threshold or flow-dependent resistance against inhalation (inspiratory muscle training [IMT]) and/or exhalation (expiratory muscle training [EMT]) [10] to stimulate this musculature and to produce changes in the muscles’ structure. The diversity of potential applications for IMT makes it impossible to provide specific guidance for all potential applications. However, there are major classes of diseases or conditions where studies support a beneficial effect of RMT on clinical outcomes in subgroups of patients: cardiorespiratory, neuromuscular, surgical, healthy aging. The misconception remains that only patients with evidence of inspiratory muscle weakness or ventilatory limitation can benefit from IMT. However, even when inspiratory muscle weakness is not an inclusion criterion, improvements in dyspnea and exercise capacity still occur after IMT [11]. To date, no reports of adverse events after IMT have been found, but there is a theoretical risk of barotrauma-related events. However, in patients with coronary artery disease, it is reasonable to minimize hypocapnia by using a slower respiratory rate. In patients who have experienced an acute exacerbation or chest infection, clinical judgment should be used regarding the risk of inducing hyperfatigue of inspiratory muscles. In this situation, it may be appropriate to reduce the intensity and/or frequency of IMT use. The vast majority of patients undergoing RMT are those with long-term chronic conditions such as respiratory disease, heart failure, obesity, and neuromuscular disease. For these patients, the timing of IMT will depend on disease management policies. RMT can be provided in the patient's home as part of primary care provided by a specialist nurse or physiotherapist or in an outpatient setting. Evidence suggests that RMT can be used both as a stand-alone intervention and as part of a multidimensional rehabilitation program in a wide range of patients. Furthermore, comorbidities that preclude physical training are not a barrier to RMT, thus making it an ideal intervention for patients with severe impairment [12].
However, despite the fact that certain effects of RMT have been shown in previous research on several populations (e.g. stroke survivors [13, 14], respiratory [11, 15,16,17], cardiac [18, 19], or cancer patients [20]), evidence regarding the efficacy of respiratory muscle training is still inconclusive and controversial. RMT reduce the occurrence of respiratory complications in stroke survivors immediately or even 3–12 months after treatment initiation [21], however evidence regarding change in swallowing function after stroke remains lacking [22]. Inspiratory muscle training benefits were demonstrated on quality of life, dyspnea, and exercise capacity in patients with pulmonary dysfunctions [4]. Regarding cardiac patients it has been shown that inspiratory muscle training improves diaphragmatic mobility in patients following cardiac surgery [23], likewise improve pulmonary function, such as FVC [24]. Little is known about clinical efficacy of RMT in cancer patients. Conflicting results have been reported regarding increases in respiratory muscle strength after lung cancer surgery and oxygenation [25]. Although, IMT and aerobic exercise improves muscle strength and exercise capacity in lung cancer patients [26]. Furthermore, the assessment of training efficacy from most work is often limited to measures of lung function and/or physical performance. Therefore, we decided to perform a systematic review along with a meta-analysis on the effectiveness of respiratory muscle training in internal and central nervous system disorders, evaluating the combined effect on respiratory function, exercise capacity and quality of life.
Methods
The study design was set as a systematic review and meta-analysis and was conducted according to the PRISMA 2020 guidelines [27]. The protocol was prospective registered in the PROSPERO database (registration number: CRD42020216639) on 07.12.2020.
Electronic searches
Publications were searched in PubMed, Embase, Web of Science, Scopus, and the Cochrane Library. The last search was launched on 8th February 2021. A detailed description of the search strategy is presented in the supplementary materials (see Appendix 1).
Study selection
In this review, we included (1) publications designed as Randomized Controlled Trial (RCT), with (2) hospitalized adults patients (i.e. > 18 years) with pulmonary dysfunction caused by an internal disease or central nervous system disorder, (3) an intervention defined as RMT (either IMT or EMT) compared to all other treatments or no intervention, and (4) with the assessment of exercise capacity, respiratory function and quality of life as primary outcomes. The inclusion criteria of device type and training characteristics were not considered in order to include the largest number of studies. A similar approach was applied to the choice of intervention location (inpatient, home-based, outpatient). The review included publications in English, Italian and Polish. Grey literature was not searched in this review. Conversely, we excluded non-RCTs and studies involving healthy adults, children or participants with sleep disorders. Furthermore, we excluded studies in which all the three outcomes (i.e. exercise capacity, respiratory function and quality of life) were not assessed. For study selection through abstract screening, two reviewers, independently, screened studies that were identified through the electronic search engines already mentioned, based on title and abstract, using the free tool Rayyan (https://rayyan.qcri.org/). A third reviewer was selected to solve any disagreements. At the end of this process, full text of the articles were obtained, and the same procedures were used for full text screening and for the assessment of the methodological quality (risk of bias assessment).
Outcomes
We aimed to assess three main outcomes, i.e. respiratory function, exercise capacity and quality of life. We assessed respiratory function with parameters of respiratory muscle weakness (i.e. FEV1, FVC, MIP) and exercise capacity with the 6-min walking test (6MWT) or other scales that aim to assess exercise capacity. Finally, we wanted to evaluate the improvement of perceived-quality of life by using questionnaires that assess changes in the perception of quality of life (e.g. St. George respiratory questionnaire, SF-36).
Data extraction and management
Screening of research records was conducted with the procedure already mentioned, then a full-text screening was conducted with the same procedure. A data extraction form was filled with all the relevant data, i.e.: authors and year of publication, number of participants and their characteristics, type of interventions and training, outcome measures and conclusions drawn by authors.
Assessment of risk of bias in included studies
Studies included in the review underwent a methodological quality assessment for risk of bias using the Cochrane Risk of Bias Tool [28]. We evaluated the following domains: (1) Selection bias: random sequence generation; (2) Selection bias: allocation concealment; (3) Performance bias: blinding of participants and personnel (4) Detection bias: blinding of outcome assessment; (5) Attrition bias: incomplete outcome data; and (6) reporting bias: selective reporting. We coded risk of bias for each domain as “high risk”, in case of a high possibility in the occurrence of bias; “low risk”, in case of a low possibility of bias; “unclear risk”, when we could not exactly define the real incidence of bias. Finally, potential publication bias was explored by visual inspection of funnel plots.
Data synthesis and statistical analysis
We used Review Manager 5.3 to conduct review, to assess the methodological quality of trials through the risk of bias tables, and for statistical analysis. We attempted to categorize the included interventions along three outcomes group: (1) respiratory function, (2) exercise capacity and (3) quality of life. Treatment effects were evaluated using Mean Difference (MD) for homogeneous outcome measures or Standardized Mean Difference (SMD) for the outcomes evaluated with different scales. Confidence Interval (CI) for continuous outcomes was identified at 95%. Statistical heterogeneity was assessed with the I2 statistic. We deemed to perform the analyses with 95% CI, based on random effects model, as we assumed that there would be the presence of heterogeneity due to the different population included in the review and the different types of training provided [29]. We planned a subgroup analysis in relation to medical diagnosis (i.e. internal diseases, pulmonary diseases, stroke and leukemia). In the case of no data available for synthesis, an email was sent to the corresponding author. We assumed a 2-week waiting period for a response.
Results
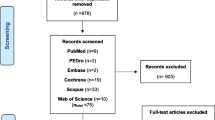
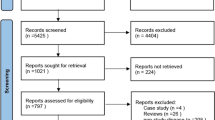
Our search identified 2220 results from 5 electronic databases. The following numbers were retrieved from publications in each of the databases: PubMed: 166; Scopus: 1596; Cochrane Library: 145; Web of Science: 44, and Embase: 269. After removing 349 duplicates, 1851 abstracts remained for screening. We excluded 1818 records with unrelated target topics and then assessed for eligibility a total of 33 full text articles. The main reasons for rejecting abstracts were: different study design than specified in the inclusion criterion and different study population. After full-text screening, 29 studies met the inclusion criteria for qualitative analysis. At the end of the process, 25 studies remained for quantitative analysis, as 4 studies did not report data as Mean and Standard Deviations. The PRISMA flowchart of the review process is shown in Fig. 1.
Characteristics of included studies
All the included studies were RCTs focusing on the use of RMT for pulmonary dysfunction. Among the 29 included studies, 8 included participants with internal diseases [30,31,32,33,34,35,36,37], 19 studies focused on the treatment of patients with pulmonary diseases [2, 38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] and the remaining 2 studies included patients with stroke and leukemia, respectively [56, 57]. The overall number of participants included within trials was 1155, with 619 patients involved in RMT programs and 536 patients treated in control groups.
With regard to the intervention provided, all the included studies used a threshold-loading device. Only one study investigated the effects of EMT on lung function, exercise tolerance, symptoms and health-related quality of life in severe COPD patients [52], whereas 5 studies evaluated the effectiveness of RMT for patients with Restrictive Thoracic Disease [41], stroke [56], leukemia [57] and COPD [47, 48]. The remaining 23 studies provided IMT programs, in order to evaluate their efficacy, in patients with COPD [2, 38,39,40, 42, 44, 45, 50, 51, 53, 54], asthma [43], lung cancer [46], bronchiectasis [49], in patients with chronic airflow limitation [55], heart failure [31, 33, 34, 37], in patients who underwent coronary artery bypass graft surgery [32, 36] and Heart Valve Replacement Surgery [30], and in patients with pulmonary arterial hypertension [35].
Three studies compared the experimental intervention to placebo intervention [30, 31, 53], whereas eight studies used sham training as comparator [35, 40, 41, 47, 50, 52, 54, 55]. In 4 studies the experimental intervention was compared to no intervention [42, 44, 45, 48] and 12 studies compared the use of RMT to conventional rehabilitation [2, 32, 33, 36,37,38,39, 43, 46, 49, 56, 57]. Sadek et al. tested the combination of high-intensity aerobic interval training and IMT, and compared it to IMT alone, high-intensity aerobic interval training alone and no intervention [34]. Finally, Majewska-Pulsakowska et al. compared the combination of IMT and cycle ergometer to IMT alone, cycle ergometer alone and no treatment [51].
The dose of therapy varies between studies, ranging from a total of 20 sessions over 10 days of treatment to a maximum of 288 sessions over 1 year.
A detailed description of the characteristics of included studies is presented in supplementary material (Table 1).
Excluded studies
We deemed 4 studies to be excluded, as in two studies no resistance training was provided in the experimental group [58, 59] and in one study the minimum load of the device (9 cmH2O) was kept constant during the study period in the control group [60]. One more study was excluded because the outcome quality of life was not investigated [61].
Risk of bias in included studies
Figure 2 shows the risk of bias in the included studies.
-
Random sequence generation (selection bias): Sixteen studies were assessed with a low risk of bias, as the authors described a random component in the sequence-generation process, whereas two studies were judged with a high risk of bias, as randomization procedures were not appropriate. The remaining 11 studies were judged with an unclear risk of bias, as no information was provided.
-
Allocation concealment (selection bias): Ten studies had a low risk of bias in this domain, as the allocation methods used were appropriate. In 19 studies there was no information about allocation concealment procedures, resulting in an unclear risk of bias.
-
Blinding of participants and personnel (performance bias): Seven studies clearly stated that participants and therapists were blinded to intervention group allocation, whereas in other 7 studies no blinding was provided. It was unclear whether participants and therapists were blinded to intervention group allocation in 15 studies.
-
Blinding of outcome assessment (detection bias): In 16 studies the outcome assessor was unaware of the participants’ assigned interventions, so the risk of bias was low. In 13 studies the risk was unclear due to lack of information.
-
Incomplete outcome data (attrition bias): Twenty-five studies were assessed with a low risk of bias for this domain, as no missing data were found. Only one study had an unclear risk of bias because information was not reported and potential missing data were not provided. Outcome data were incomplete in the remaining 2 studies.
-
Selective reporting (reporting bias): In 8 studies the risk of bias was low, whereas it was unclear whether selective reporting occurred in 19 studies, as the study protocols were not available. In 2 studies selective reporting was identified.
Effects of intervention
Comparison 1 quality of life
Twenty-one studies were analyzed for quality of life, with a total of 772 participants. Subgroup analyses were performed, based on medical diagnosis (i.e. internal diseases, pulmonary diseases and leukemia). The analyses were performed using Standardized Mean Difference (SMD) with random effects model, since all the included studies used different outcome measures for the same outcome and we expected high values of heterogeneity. The total result showed a significant difference in favour of RMT (SMD = 0.60, 95% CI 0.30 to 0.91, I2 = 74%), as well as for all the subgroups, i.e. internal diseases (SMD = 0.76, 95% CI 0.08 to 1.44, I2 = 82%), pulmonary diseases (SMD = 0.41, 95% CI 0.11 to 0.71, I2 = 58%) and leukemia (SMD = 1.74, 95% CI 0.92–2.56, I2 = N/A) (Fig. 3). Figure 3 shows the funnel plot of included studies.
Comparison 2 exercise capacity
A total of 24 studies were included and divided in four subgroups (i.e. internal diseases, pulmonary diseases, stroke and leukemia). The overall number of participants was 853 and analyses were performed with SMD and random effects model. Results showed a significant improvement in exercise capacity in participants who underwent RMT (SMD = 0.58, 95% CI 0.33–0.84, I2 = 67%). The same results were obtained for internal diseases (SMD = 0.99, 95% CI 0.43–1.55, I2 = 72%), pulmonary diseases (SMD = 0.25, 95% CI 0.04–0.47, I2 = 31%), stroke (SMD = 1.05, 95% CI 0.28–1.83, I2 = N/A) and for leukemia patients (SMD = 1.38, 95% CI 0.60–2.17, I2 = N/A) (Fig. 4). Figure 4 shows the funnel plot of the Comparison 2.
Comparison 3 respiratory function–FEV1
Twelve studies with 374 participants were analyzed. Analyses were performed with SMD, as studies assessed respiratory function by using FEV1 values but with different unit of measure. No significant difference was found between the two treatments (SMD = 0.11, 95% CI − 0.21–0.43, I2 = 56%), as well as for the subgroups internal diseases (SMD = 0.00, 95% CI − 0.61–0.61, I2 = 59%) and pulmonary diseases (SMD = 0.02, 95% CI − 0.33–0.37, I2 = 41%). A significant improvement in respiratory function assessed with FEV1 was found in stroke patients (SMD = 1.23, 95% CI 0.44–2.02, I2 = N/A) (Fig. 5). Figure 5 shows the funnel plot of the Comparison 3.
Comparison 4 respiratory function—MIP
A total of 16 studies with 557 participants were included in the analysis, which were performed with SMD with random effects model. RMT resulted to be more effective than control condition (SMD = 0.89, 95% CI 0.56–1.22, I2 = 66%). Significant differences can be found in both internal diseases (SMD = 1.28, 95% CI 0.62–1.95, I2 = 65%) and pulmonary diseases subgroups (SMD = 0.78, 95% CI 0.43–1.13, I2 = 57%), but not in stroke subgroup (SMD = 0.00, 95% CI − 0.72–0.72, I2 = N/A) (Fig. 6). Figure 6 shows the funnel plot of the included studies.
Comparison 5 respiratory function—FVC
Eight studies and 252 participants were included. Analyses were performed using SMD with fixed effect model. Final result showed a significant difference between RMT and control condition (SMD = 0.32, 95% CI 0.07–0.57, I2 = 0%), but subgroup analyses revealed no difference between interventions for internal diseases (SMD = 0.18, 95% CI − 0.21–0.57, I2 = 47%), pulmonary diseases (SMD = 0.36, 95% CI − 0.00–0.73, I2 = 0%) and for stroke patients (SMD = 0.65, 95% CI − 0.09–1.39, I2 = N/A) (Fig. 7). Figure 7 shows the funnel plot of the included studies.
Discussion
This review aimed to evaluate the effectiveness of RMT in internal and central nervous system disorders, and its effect on pulmonary function, exercise capacity and quality of life. Results suggest that patients with internal and central nervous system disorders who underwent RMT had better quality of life and improved significantly their performance in exercise capacity and in respiratory function assessed with FVC and MIP when compared to control conditions (i.e. no intervention, sham training, placebo or conventional treatments). No difference was found in respiratory function assessed with FEV1. However, results need to be considered carefully because of the following limitations.
In this systematic review, grey literature was not searched, in order to be as specific as possible, given also the large quantity of records obtained from the search in the primary databases. Furthermore, most of the analyses showed moderate-to-high values of heterogeneity, highlighting the presence of important inconsistency. The Cochrane Handbook for Systematic Reviews of Interventions suggests consideration of several possible sources of heterogeneity and asymmetry in funnel plots [29]. Indeed, the likelihood of drawing correct inferences from a meta-analysis decreases with increasing heterogeneity [62], thus investigation and plausible explanations for the presence of heterogeneity need to be carried out.
Results on quality of life, exercise capacity and respiratory function (FEV1 and MIP), are affected by clinical heterogeneity, likely due to difference in type and doses of intervention and comparator. In relation to the dose of therapy provided, this ranged from a total of 20 sessions over 10 days of treatment, to a maximum of 288 sessions over 1 year, ranging from twice a day all week to once a day twice a week. Likewise, in terms of training intensity, differences were present among the included studies. Respiratory resistance of the subjects ranged between 15 and 60% of the MIP. The included studies also varied in the devices used, including i.e. POWERbreathe, Threshold IMT, Respifit-S. This result tells us that a lack of agreement exists on the standard and/or optimal dose of therapy to deliver for achieving significant improvement, to patients with internal and central nervous system disorders. Furthermore, after analyzing the funnel plot of the Comparison 1, we observed a lack of studies in favour of RMT, suggesting the possibility of publication bias underestimating the effect of the experimental treatment, within the pulmonary diseases subgroup.
In Comparison 3 (Respiratory function—FEV1) selective outcome reporting affected the final results. In depth, we found that studies with unclear or high risk of bias for missing outcome data were less precise, underestimating significantly the effect of RMT on FEV1. Excluding these articles from the meta-analyses, results changed significantly in favour of RMT. This lack of methodology in trials conducting and reporting represents a major flaw in current literature in the field. Thus, it is essential that future trials reports will adhere to acknowledged standards, such as the CONSORT statement [63]. Furthermore, an evidence of imprecision in the effect estimate is observed, especially in Comparison 3. Most of the included studies had similar effects both in favour of the experimental or control treatment, making it difficult to draw a firm conclusion on the effectiveness of RMT.
The RMT resulted to be advantageous especially for the improvement of respiratory function assessed with FVC. In this case, the absence of heterogeneity and the significant results in favour of RMT provide evidence in favour of such training for patients with pulmonary dysfunction, nevertheless better training programs with standard dose of therapy need to be implemented, together with better trial reporting.
Comparing our results to previous research, it was indicated that RMT is effective to reduce postoperative pulmonary complications and length of hospital stay in patients undergoing surgery [64, 65]. Thus, the enhancement in the post-hospitalization phase of rehabilitation with RMT seems to bring additional benefits conveying to physical performance and quality of life. However, in line with the previous meta-analysis, the results also suggested no significant effect of RMT on FEV1 and the marginal on FVC [66]. Thus, it seems likely that elastic recoil and lung tissue properties, rather than expiratory muscle strength, may determine the maximum expiratory flow. This, in turn, is related to the pathomechanism of respiratory muscle weakness caused by lung tissue changes, or weakness due to skeletal muscle weakness. It is assumed that combined expiratory and inspiratory training may have a greater effect on lung function by increasing inspiratory reserve volume and elastic recoil and yielding significant improvements in FEV1 [14]. Therefore, it might be presumed that the patients had better perception of improvements at the level of activity, participation and quality of life, rather than structure and function (ventilator) aspects of the lung. Moreover, the novelty of this meta-analysis lies in the summarization of the effectiveness of RMT in several health areas in different patient groups. To our knowledge, a review with different disease classifications has not been performed previously. This approach was driven by an attempt to estimate the results obtained to the population of patients after hospitalization for COVID-19.
One should also consider the results obtained in the context of the current pandemic situation, with the first published clinical trials indicating the effectiveness of RMT on pulmonary functions, dyspnea, functional performance and QOL, after weaning from mechanical ventilation [67]. Although the role of RMT in mitigating the respiratory complications of viral infection has not yet been confirmed, the available evidence seems to suggest that this training may help reduce the risk of serious complications during viral infection [68]. It is also worth noting that RMT can be performed independently by the patient at home [69]. This opens many possibilities for the implementation of telerehabilitation or using new technologies [70]. Our team is currently running an ERS-funded project under Long-Term Research Fellowships. This project produced software to perform RMT in virtual reality. Based on our previous observations, we hypothesize that the virtual environment will lead to stress reduction during therapy [71,72,73]. Although the clinical experiment is currently in the implementation phase, it seems that lowering stress levels in patients after COVID-19 hospitalization may be one of the pillars of comprehensive pulmonary rehabilitation.
Conclusion
Respiratory muscle training seems to be more effective than control conditions (i.e. no intervention, sham training, placebo or conventional treatment), in patients with pulmonary dysfunction due to internal and central nervous system disorders, for quality of life, exercise capacity and respiratory function assessed with MIP and FVC, but not with FEV1. However, standardized training programs with optimal dose of therapy need to be developed, and better trial reporting need to be implemented.
Data availability
The data presented in this study are available on request from the corresponding author.
Change history
25 July 2022
Missing Open Access funding information has been added in the Funding Note.
References
Oliveira, M. J. P., Rodrigues, F., Firmino-Machado, J., Ladeira, I. T., Lima, R., Conde, S. D., & Guimaraes, M. (2018). Assessment of respiratory muscle weakness in subjects with neuromuscular disease. Respiratory Care, 63(10), 1223–1230.
Ahmad, H., Justine, M., Othman, Z., Mohan, V., & Mirza, F. T. (2013). The outcomes of short term inspiratory muscle training (IMT) combined with chest physiotherapy in hospitalized COPD patients. Bangladesh Journal of Medical Science, 12(4), 398–404.
Menezes, K. K., Nascimento, L. R., Avelino, P. R., Alvarenga, M. T. M., & Teixeira-Salmela, L. F. (2018). Efficacy of interventions to improve respiratory function after stroke. Respiratory Care, 63(7), 920–933.
Gosselink, R., De Vos, J., van den Heuvel, S. P., Segers, J., Decramer, M., & Kwakkel, G. (2011). Impact of inspiratory muscle training in patients with COPD: What is the evidence? European Respiratory Journal, 37(2), 416–425.
Manifield, J., Winnard, A., Hume, E., Armstrong, M., Baker, K., Adams, N., Vogiatzis, I., & Barry, G. (2021). Inspiratory muscle training for improving inspiratory muscle strength and functional capacity in older adults: A systematic review and meta-analysis. Age and Ageing, 50(3), 716–724.
Polese, J. C., Pinheiro, M. B., Faria, C. D., Britto, R. R., Parreira, V. F., & Teixeira-Salmela, L. F. (2013). Strength of the respiratory and lower limb muscles and functional capacity in chronic stroke survivors with different physical activity levels. Brazilian Journal of Physical Therapy, 17(5), 487–493.
Bott, J., Blumenthal, S., Buxton, M., Ellum, S., Falconer, C., Garrod, R., Harvey, A., Hughes, T., Lincoln, M., Mikelsons, C., Potter, C., Pryor, J., Rimington, L., Sinfield, F., Thompson, C., Vaughn, P., White, J., British Thoracic Society Physiotherapy Guideline Development Group. (2009). Guidelines for the physiotherapy management of the adult, medical, spontaneously breathing patient. Thorax, 64(Suppl 1), i1-51.
Smith, K., Cook, D., Guyatt, G. H., Madhavan, J., & Oxman, A. D. (1992). Respiratory muscle training in chronic airflow limitation: A meta-analysis. The American Review of Respiratory Disease, 145(3), 533–539.
Pozuelo-Carrascosa, D. P., Carmona-Torres, J. M., Laredo-Aguilera, J. A., Latorre-Roman, P. A., Parraga-Montilla, J. A., & Cobo-Cuenca, A. I. (2020). Effectiveness of respiratory muscle training for pulmonary function and walking ability in patients with stroke: A systematic review with meta-analysis. International Journal of Environmental Research and Public Health. https://doi.org/10.3390/ijerph17155356
Battaglia, E., Fulgenzi, A., & Ferrero, M. E. (2009). Rationale of the combined use of inspiratory and expiratory devices in improving maximal inspiratory pressure and maximal expiratory pressure of patients with chronic obstructive pulmonary disease. Archives of Physical Medicine and Rehabilitation, 90(6), 913–918.
Lotters, F., van Tol, B., Kwakkel, G., & Gosselink, R. (2002). Effects of controlled inspiratory muscle training in patients with COPD: A meta-analysis. European Respiratory Journal, 20(3), 570–576.
Ozsoy, I., Kahraman, B. O., Ozsoy, G., Ilcin, N., Tekin, N., & Savci, S. (2021). Effects of an integrated exercise program including “functional” inspiratory muscle training in geriatric individuals with and without chronic obstructive pulmonary disease. Annals of Geriatric Medicine and Research, 25(1), 45–54.
Xiao, Y., Luo, M., Wang, J., & Luo, H. (2012). Inspiratory muscle training for the recovery of function after stroke. Cochrane Database Systematic Reviews. https://doi.org/10.1002/14651858.CD009360.pub2
Gomes-Neto, M., Saquetto, M. B., Silva, C. M., Carvalho, V. O., Ribeiro, N., & Conceicao, C. S. (2016). Effects of respiratory muscle training on respiratory function, respiratory muscle strength, and exercise tolerance in patients poststroke: A systematic review with meta-analysis. Archives of Physical Medicine and Rehabilitation, 97(11), 1994–2001.
Martín-Valero, R., Jimenez-Cebrian, A. M., Moral-Munoz, J. A., de la Casa-Almeida, M., Rodriguez-Huguet, M., & Casuso-Holgado, M. J. (2020). The efficacy of therapeutic respiratory muscle training interventions in people with bronchiectasis: A systematic review and meta-analysis. Journal of Clinical Medicine. https://doi.org/10.3390/jcm9010231
Beaumont, M., Forget, P., Couturaud, F., & Reychler, G. (2018). Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis. The Clinical Respiratory Journal, 12(7), 2178–2188.
Zhang, W., Wang, Q., Liu, L., Yang, W., & Liu, H. (2020). Effects of physical therapy on lung function in children with asthma: A systematic review and meta-analysis. Pediatric Research. https://doi.org/10.1038/s41390-020-0874-x
Azambuja, A. C. M., de Oliveira, L. Z., & Sbruzzi, G. (2020). Inspiratory muscle training in patients with heart failure: What is new? Systematic review and meta-analysis. Physical Therapy, 100(12), 2099–2109.
Martin-Valero, R., De La Casa Almeida, M., Casuso-Holgado, M. J., & Heredia-Madrazo, A. (2015). Systematic review of inspiratory muscle training after cerebrovascular accident. Respiratory Care, 60(11), 1652–1659.
Rosero, I. D., Ramirez-Velez, R., Lucia, A., Martinez-Velilla, N., Santos-Lozano, A., Valenzuela, P. L., Morilla, I., & Izquierdo, M. (2019). Systematic review and meta-analysis of randomized, controlled trials on preoperative physical exercise interventions in patients with non-small-cell lung cancer. Cancers (Basel). https://doi.org/10.3390/cancers11070944
Menezes, K. K., Nascimento, L. R., Ada, L., Polese, J. C., Avelino, P. R., & Teixeira-Salmela, L. F. (2016). Respiratory muscle training increases respiratory muscle strength and reduces respiratory complications after stroke: A systematic review. Journal of Physiotherapy, 62(3), 138–144.
Zhang, W., Pan, H., Zong, Y., Wang, J., & Xie, Q. (2021). Respiratory muscle training reduces respiratory complications and improves swallowing function after stroke: A systematic review and meta-analysis. Archives of Physical Medicine and Rehabilitation. https://doi.org/10.1016/j.apmr.2021.10.020
Kodric, M., Trevisan, R., Torregiani, C., Cifaldi, R., Longo, C., Cantarutti, F., & Confalonieri, M. (2013). Inspiratory muscle training for diaphragm dysfunction after cardiac surgery. Journal of Thoracic and Cardiovascular Surgery, 145(3), 819–823.
Gayan-Ramirez, G., Gosselin, N., Troosters, T., Bruyninckx, F., Gosselink, R., & Decramer, M. (2008). Functional recovery of diaphragm paralysis: A long-term follow-up study. Respiratory Medicine, 102(5), 690–698.
Brocki, B. C., Andreasen, J. J., Langer, D., Souza, D. S., & Westerdahl, E. (2016). Postoperative inspiratory muscle training in addition to breathing exercises and early mobilization improves oxygenation in high-risk patients after lung cancer surgery: A randomized controlled trial. European Journal of Cardio-Thoracic Surgery, 49(5), 1483–1491.
Liu, J. F., Kuo, N. Y., Fang, T. P., Chen, J. O., Lu, H. I., & Lin, H. L. (2021). A six-week inspiratory muscle training and aerobic exercise improves respiratory muscle strength and exercise capacity in lung cancer patients after video-assisted thoracoscopic surgery: A randomized controlled trial. Clinical Rehabilitation, 35(6), 840–850.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hrobjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372, n71.
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., Savovic, J., Schulz, K. F., Weeks, L., Sterne, J. A., Cochrane Bias Methods Group, Cochrane Statistical Methods Group. (2011). The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 343, d5928.
Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., & VA, W. (2021). Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane, 2021. Retrieved from www.training.cochrane.org/handbook.
Cargnin, C., Karsten, M., Guaragna, J. C. V. D. C., & Dal Lago, P. (2019). Inspiratory muscle training after heart valve replacement surgery improves inspiratory muscle strength, lung function, and functional capacity: A randomized controlled trial. Journal of Cardiopulmonary Rehabilitation and Prevention, 39(5), E1–E7.
Dall’Ago, P., Chiappa, G. R., Guths, H., Stein, R., & Ribeiro, J. P. (2006). Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: A randomized trial. Journal of the American College of Cardiology, 47(4), 757–763.
Miozzo, A. P., Stein, C., Marcolino, M. Z., Sisto, I. R., Hauck, M., Coronel, C. C., & Plentz, R. D. M. (2018). Effects of high-intensity inspiratory muscle training associated with aerobic exercise in patients undergoing CABG: Randomized clinical trial. Brazilian Journal of Cardiovascular Surgery, 33(4), 376–383.
Palau, P., Domínguez, E., Núñez, E., Schmid, J. P., Vergara, P., Ramón, J. M., Mascarell, B., Sanchis, J., Chorro, F. J., & Núñez, J. (2014). Effects of inspiratory muscle training in patients with heart failure with preserved ejection fraction. European Journal of Preventive Cardiology, 21(12), 1465–1473.
Sadek, Z., Salami, A., Youness, M., Awada, C., Hamade, M., Joumaa, W. H., Ramadan, W., & Ahmaidi, S. (2020). A randomized controlled trial of high-intensity interval training and inspiratory muscle training for chronic heart failure patients with inspiratory muscle weakness. Chronic Illness. https://doi.org/10.1177/1742395320920700
Saglam, M., Arikan, H., Vardar-Yagli, N., Calik-Kutukcu, E., Inal-Ince, D., Savci, S., Akdogan, A., Yokusoglu, M., Kaya, E. B., & Tokgozoglu, L. (2015). Inspiratory muscle training in pulmonary arterial hypertension. Journal of Cardiopulmonary Rehabilitation and Prevention, 35(3), 198–206.
Savci, S., Degirmenci, B., Saglam, M., Arikan, H., Inal-Ince, D., Turan, H. N., & Demircin, M. (2011). Short-term effects of inspiratory muscle training in coronary artery bypass graft surgery: A randomized controlled trial. Scandinavian Cardiovascular Journal, 45(5), 286–293.
Winkelmann, E. R., Chiappa, G. R., Lima, C. O., Viecili, P. R., Stein, R., & Ribeiro, J. P. (2009). Addition of inspiratory muscle training to aerobic training improves cardiorespiratory responses to exercise in patients with heart failure and inspiratory muscle weakness. American Heart Journal, 158(5), 768.e761–767.
Arnedillo, A., Gonzalez-Montesinos, J. L., Fernandez-Santos, J. R., Vaz-Pardal, C., España-Domínguez, C., Ponce-González, J. G., & Cuenca-García, M. (2020). Effects of a rehabilitation programme with a nasal inspiratory restriction device on exercise capacity and quality of life in COPD. International Journal of Environmental Research and Public Health. https://doi.org/10.3390/ijerph17103669
Beaumont, M., Forget, P., Couturaud, F., & Reychler, G. (2018). Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis. Clinical Respiratory Journal, 12(7), 2178–2188.
Beckerman, M., Magadle, R., Weiner, M., & Weiner, P. (2005). The effects of 1 year of specific inspiratory muscle training in patients with COPD. Chest, 128(5), 3177–3182.
Budweiser, S., Moertl, M., Jörres, R. A., Windisch, W., Heinemann, F., & Pfeifer, M. (2006). Respiratory muscle training in restrictive thoracic disease: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation, 87(12), 1559–1565.
Chuang, H. Y., Chang, H. Y., Fang, Y. Y., & Guo, S. E. (2017). The effects of threshold inspiratory muscle training in patients with chronic obstructive pulmonary disease: A randomised experimental study. Journal of clinical nursing, 26(23–24), 4830–4838.
Duruturk, N., Acar, M., & Doğrul, M. I. (2018). Effect of inspiratory muscle training in the management of patients with asthma: A randomized controlled trial. Journal of cardiopulmonary rehabilitation and prevention, 38(3), 198–203.
Elmorsi, A. S., Eldesoky, M. E., Mohsen, M. A. A., Shalaby, N. M., & Abdalla, D. A. (2016). Effect of inspiratory muscle training on exercise performance and quality of life in patients with chronic obstructive pulmonary disease. Egyptian Journal of Chest Diseases and Tuberculosis, 65(1), 41–46.
Garcia, S., Rocha, M., Pinto, P., Lopes, A. M. F., & Bárbara, C. (2008). Inspiratory muscle training in COPD patients. Revista Portuguesa de Pneumologia, 14(2), 177–194.
Huang, J., Lai, Y., Zhou, X., Li, S., Su, J., Yang, M., & Che, G. (2017). Short-term high-intensity rehabilitation in radically treated lung cancer: A three-armed randomized controlled trial. Journal of Thoracic Disease, 9(7), 1919–1929.
Koppers, R. J. H., Vos, P. J. E., Boot, C. R. L., & Folgering, H. T. M. (2006). Exercise performance improves in patients with COPD due to respiratory muscle endurance training. Chest, 129(4), 886–892.
Leelarungrayub, J., Pinkaew, D., Puntumetakul, R., & Klaphajone, J. (2017). Effects of a simple prototype respiratory muscle trainer on respiratory muscle strength, quality of life and dyspnea, and oxidative stress in COPD patients: A preliminary study. International Journal of COPD, 12, 1415–1425.
Liaw, M. Y., Wang, Y. H., Tsai, Y. C., Huang, K. T., Chang, P. W., Chen, Y. C., & Lin, M. C. (2011). Inspiratory muscle training in bronchiectasis patients: A prospective randomized controlled study. Clinical Rehabilitation, 25(6), 524–536.
Magadle, R., McConnell, A. K., Beckerman, M., & Weiner, P. (2007). Inspiratory muscle training in pulmonary rehabilitation program in COPD patients. Respiratory Medicine, 101(7), 1500–1505.
Majewska-Pulsakowska, M., Wytrychowski, K., & Rożek-Piechura, K. (2016). The role of inspiratory muscle training in the process of rehabilitation of patients with chronic obstructive pulmonary disease. Advances in Experimental Medicine and Biology, 885, 47–51.
Mota, S., Guell, R., Barreiro, E., Solanes, I., Ramirez-Sarmiento, A., Orozco-Levi, M., Casan, P., Gea, J., & Sanchis, J. (2007). Clinical outcomes of expiratory muscle training in severe COPD patients. Respiratory Medicine, 101(3), 516–524.
Riera, H. S., Rubio, T. M., Ruiz, F. O., Ramos, P. C., Del Castillo Otero, D., Hernandez, T. E., & Gomez, J. C. (2001). Inspiratory muscle training in patients with COPD: Effect on dyspnea, exercise performance, and quality of life. Chest, 120(3), 748–756.
Schultz, K., Jelusic, D., Wittmann, M., Kramer, B., Huber, V., Fuchs, S., Lehbert, N., Wingart, S., Stojanovic, D., Gohl, O., Alma, H. J., de Jong, C., van der Molen, T., Faller, H., & Schuler, M. (2018). Inspiratory muscle training does not improve clinical outcomes in 3-week COPD rehabilitation: Results from a randomised controlled trial. European Respiratory Journal. https://doi.org/10.1183/13993003.02000-2017
Serón, P., Riedemann, P., Muñoz, S., Doussoulin, A., Villarroel, P., & Cea, X. (2005). Effect of inspiratory muscle training on muscle strength and quality of life in patients with chronic airflow limitation: A randomized controlled trial. Archivos de Bronconeumologia, 41(11), 601–606.
Sutbeyaz, S. T., Koseoglu, F., Inan, L., & Coskun, O. (2010). Respiratory muscle training improves cardiopulmonary function and exercise tolerance in subjects with subacute stroke: A randomized controlled trial. Clinical Rehabilitation, 24(3), 240–250.
El-Nahas, N. G., & Abdeen, H. A. (2019). Respiratory training efficacy on quality of life and functional capacity in patients with Leukemia. Journal of Advanced Pharmacy Education and Research, 9(2), 46–52.
Jastrzebski, D., Maksymiak, M., Kostorz, S., Bezubka, B., Osmanska, I., Mlynczak, T., Rutkowska, A., Baczek, Z., Ziora, D., & Kozielski, J. (2015). Pulmonary rehabilitation in advanced lung cancer patients during chemotherapy (Vol. 861, pp. 57–64). Springer International Publishing.
Lin, W. C., Yuan, S. C., Chien, J. Y., Weng, S. C., Chou, M. C., & Kuo, H. W. (2012). The effects of respiratory training for chronic obstructive pulmonary disease patients: A randomised clinical trial. Journal of Clinical Nursing, 21(19–20), 2870–2878.
dos Santos, T. D., Pereira, S. N., Portela, L. O. C., Cardoso, D. M., Lago, P. D., dos Santos Guarda, N., Moresco, R. N., Pereira, M. B., & de Albuquerque, I. M. (2019). Moderate-to-high intensity inspiratory muscle training improves the effects of combined training on exercise capacity in patients after coronary artery bypass graft surgery: A randomized clinical trial. International Journal of Cardiology, 279, 40–46.
Zeren, M., Demir, R., Yigit, Z., & Gurses, H. N. (2016). Effects of inspiratory muscle training on pulmonary function, respiratory muscle strength and functional capacity in patients with atrial fibrillation: A randomized controlled trial. Clinical Rehabilitation, 30(12), 1165–1174.
Melsen, W. G., Bootsma, M. C. J., Rovers, M. M., & Bonten, M. J. M. (2014). The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clinical Microbiology and Infection, 20(2), 123–129.
Schulz, K. F., Altman, D. G., & Moher, D. (2010). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ, 340, c332.
Kendall, F., Oliveira, J., Peleteiro, B., Pinho, P., & Bastos, P. T. (2018). Inspiratory muscle training is effective to reduce postoperative pulmonary complications and length of hospital stay: A systematic review and meta-analysis. Disability and Rehabilitation, 40(8), 864–882.
Elkins, M., & Dentice, R. (2015). Inspiratory muscle training facilitates weaning from mechanical ventilation among patients in the intensive care unit: A systematic review. Journal of Physiotherapy, 61(3), 125–134.
Templeman, L., & Roberts, F. (2020). Effectiveness of expiratory muscle strength training on expiratory strength, pulmonary function and cough in the adult population: A systematic review. Physiotherapy, 106, 43–51.
Abodonya, A. M., Abdelbasset, W. K., Awad, E. A., Elalfy, I. E., Salem, H. A., & Elsayed, S. H. (2021). Inspiratory muscle training for recovered COVID-19 patients after weaning from mechanical ventilation: A pilot control clinical study. Medicine (Baltimore), 100(13), e25339.
Severin, R., Arena, R., Lavie, C. J., Bond, S., & Phillips, S. A. (2020). Respiratory muscle performance screening for infectious disease management following COVID-19: A highly pressurized situation. American Journal of Medicine, 133(9), 1025–1032.
Formiga, M. F., Dosbaba, F., Hartman, M., Batalik, L., Plutinsky, M., Brat, K., Ludka, O., & Cahalin, L. P. (2020). Novel versus traditional inspiratory muscle training regimens as home-based, stand-alone therapies in COPD: Protocol for a randomized controlled trial. International Journal of Chronic Obstructive Pulmonary Disease, 15, 2147–2155.
Rutkowski, S. (2021). Management challenges in chronic obstructive pulmonary disease in the COVID-19 pandemic: telehealth and virtual reality. Journal of Clinical Medicine. https://doi.org/10.3390/jcm10061261
Rutkowski, S., Szary, P., Rutkowska, A., Sacha, J., & Casaburi, R. (2020). Implementation of immersive virtual reality influences outcomes of exercise test. European Respiratory Journal. https://doi.org/10.1183/13993003.congress-2020.2879
Rutkowski, S., Szczegielniak, J., & Szczepanska-Gieracha, J. (2021). Evaluation of the efficacy of immersive virtual reality therapy as a method supporting pulmonary rehabilitation: A randomized controlled trial. Journal of Clinical Medicine, 10(2), 352.
Szczepanska-Gieracha, J., Cieslik, B., Rutkowski, S., Kiper, P., & Turolla, A. (2020). What can virtual reality offer to stroke patients? A narrative review of the literature. NeuroRehabilitation, 47(2), 109–120.
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement. Sebastian Rutkowski wish to thank the European Respiratory Society to fund a Fellowship Grant (ERS LTRF202001-00746) in San Camillo Hospital in Venice, Italy—a scientific institute specialized and internationally recognized for excellence in virtual reality rehabilitation, which has facilitated the development of this study. The APC was funded by Faculty of Physical Education and Physiotherapy of Opole University of Technology.
Author information
Authors and Affiliations
Contributions
AT, SR—Conceptualization, LC, AT, SR—methodology, LC, GP, SF, FB, AR, SR—investigation, LC, AT, SR—writing—original draft preparation, LC, AT, SR—writing—review and editing, L.C., A.T., S.R., funding acquisition, S.R. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cacciante, L., Turolla, A., Pregnolato, G. et al. The use of respiratory muscle training in patients with pulmonary dysfunction, internal diseases or central nervous system disorders: a systematic review with meta-analysis. Qual Life Res 32, 1–26 (2023). https://doi.org/10.1007/s11136-022-03133-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-022-03133-y