Abstract
Objective
To compare the impact of telerehabilitation versus conventional rehabilitation on the recovery outcomes of patients with chronic respiratory disease (CRD).
Methods
The Cochrane Library, MEDLINE, Web of Science and Embase were searched to collect randomized controlled trials (RCTs) on telerehabilitation for the rehabilitation of patients with chronic respiratory system diseases since the establishment of the database to November 14, 2023. Two researchers independently screened the literature and extracted valid data according to the inclusion criteria. The quality assessment of included studies was conducted individually by using the RoB 2(Risk of Bias 2) tool, followed by meta-analysis using RevMan5.3 software.
Results
Based on inclusion and exclusion criteria, 21 RCTs were included, comprising 3030 participants, with 1509 in the telerehabilitation group and 1521 in the conventional rehabilitation group. Meta-analysis results indicated that compared to conventional rehabilitation, video conference-based telerehabilitation demonstrated significant improvements in short-term (≤ 6 months) outcomes, including 6-min walk distance (6MWD) (MD = 7.52, 95% CI: 2.09, 12.94), modified Medical Research Council Dyspnea Scale (mMRC) (MD = -0.29, 95% CI: -0.41, -0.18), COPD assessment test (CAT) (MD = -1.77, 95% CI: -3.52, -0.02), HADS (MD = -0.44, 95% CI: -0.86, -0.03), and St. George’s Respiratory Questionnaire (SGRQ’s) activity, impact, and symptom scores. In the long term (> 6 months), although improvements persisted in 6WMD [MD = 12.89, 95% CI (-0.37, 26.14)], mMRC [MD = -0.38, 95% CI (-0.56, -0.21)], CAT [MD = -1.39, 95% CI (-3.83, 1.05)], Hospital anxiety and depression scale (HADS) [MD = -0.34, 95% CI (-0.66, -0.03)], and SGRQ’s Activity, Impact, and Symptom scores between intervention and control groups, statistically significant differences were observed only for mMRC and HADS. Without considering time factors, the intervention group exhibited some improvement in FEV1% predicted and the forced expiratory volume in the first one second (FEV1)/ forced vital capacity (FVC) (%) without statistical significance compared to the control group.
Conclusion
Telerehabilitation therapy demonstrates short-term benefits in enhancing patients’ daily activity capacity, improving respiratory function, and enhancing mental health status, thereby improving patients’ quality of life. However, further high-quality, large-sample RCTs are required to ascertain its long-term effectiveness conclusively.
Trial registration
This study protocol was approved and registered in PROSPERO: CRD 42024509154.
Similar content being viewed by others
Introduction
Chronic Respiratory Diseases (CRD) represent a significant public health issue worldwide, encompassing conditions such as Chronic Obstructive Pulmonary Disease (COPD), bronchial asthma, bronchiectasis, interstitial lung diseases, obstructive sleep apnea syndrome, and lung cancer. CRD exhibit substantial morbidity, mortality, and disability rates. Global Burden of Disease studies suggest that CRD affects approximately 545 million individuals worldwide, constituting 7.4% of the global population [1]. Findings from the 2018 China Pulmonary Health (CPH) Study reveal that COPD’s overall prevalence among individuals aged 20 and above in China stands at 8.6%, with nearly 100 million patients nationwide. Notably, prevalence rates among males (11.9%) significantly surpass those among females (5.4%), with prevalence escalating with age. Among individuals aged 40 and above, COPD prevalence skyrockets to 13.7% [2]. Furthermore, according to the latest China Disease Burden Report, COPD ranks as the third leading cause of death among Chinese residents with a mortality rate of 68 per 100,000 [3].
CRD can result in debilitating symptoms, including dyspnea, fatigue, anxiety, depression, fear. It also impairs exercise tolerance, daily functioning, reduces quality-of-life, and escalates the risk of hospitalization and mortality, imposing substantial financial burdens on healthcare systems, amounting to billions of dollars annually. Among these, COPD accounts for 56% of the costs associated with CRD, serving as the most common cause of mortality from chronic respiratory system diseases [4].
Telerehabilitation (TR) refers to the provision of online medical and health services to returning home or home-based patients through technological means such as the internet, big data, and cloud computing. It offers physical therapy, speech therapy, remote monitoring, and consultations. TR provides a novel approach to pulmonary rehabilitation for CRD patients. It not only meets their medical service needs and reduces healthcare costs but also enhances the accessibility of service offerings. It addresses challenges faced in pulmonary rehabilitation such as transportation and distance barriers, thereby offering more choices for improving healthcare and pulmonary rehabilitation services. However, there are still some obstacles to participation in TR, including severe shortages of programs due to reasons such as patients’ lack of knowledge, insufficient funds, exacerbation of disease progression, transportation issues, and inadequate institutional support [5], which prevent patients from completing TR.
Previous meta-analyses have highlighted both the advantages and disadvantages of various intervention measures. However, these studies are not without limitations, including inadequate sample sizes [6] and a lack of observation regarding their effects on depression and anxiety [7]. Furthermore, with the rapid advancement of technology and the widespread application of telerehabilitation, an updated review is needed to assess the latest evidence and draw more robust conclusions. Hence, we conducted an updated meta-analysis based on randomized controlled trials (RCTs), incorporating a greater number of original studies, expanding the sample size, and consequently enhancing the effectiveness of the tests, eventually offering novel perspectives for clinical decision-making. In line with the “evidence-based research” framework, we have reviewed all systematic reviews on this topic to ensure that our study builds on the existing body of evidence and addresses the identified gaps [8,9,10,11,12]. This approach ensures the relevance and necessity of our review in contributing valuable insights to the ongoing discourse on telerehabilitation for CRD patients.
Methods
This meta-analysis followed the guidelines outlined in the Cochrane Handbook for the Systematic Review of Interventions (for details, see at http://training.cochrane.org/handbook), as well as the Preferred Reporting Items for Systematic Review and Meta-Analyses for reporting it [13]. This study protocol was approved and registered in PROSPERO (CRD 42024509154).
Inclusion and exclusion criteria
Study type
Parallel group randomized controlled trials (RCTs).
Study participants
-
(1)
Age ≥ 18 years;
-
(2)
Patients diagnosed with CRD such as COPD, bronchiectasis, and interstitial lung disease;
-
(3)
Patients would have no major physical disabilities, could move around independently, and could participate in rehabilitation exercises and activities via remote methods.
Intervention measures
Experimental group: remote pulmonary rehabilitation, such as telemedicine video consultation, Virtual Autonomous Physiotherapist Agent, video-guided exercises, etc.
Control group: standard care (Traditional exercise rehabilitation does not rely on remote technology).
Outcome indicators
Based on the definition of CRD and the manifestation of rehabilitation effects, the following primary outcome measures were selected from both physiological function and disease symptom perspectives: 6-min walk test, St. George’s Respiratory Questionnaire (SGRQ), and modified Medical Research Council Dyspnea Scale (mMRC). Additionally, COPD Assessment Test (CAT), Hospital Anxiety and Depression Scale (HADS), and pulmonary function tests were chosen as secondary outcome measures to observe the rehabilitation effects of the two intervention methods on CRD patients.
The distance covered by the 6-min patient walking (6MWD) is shown as the results of the 6-min walk test.
Exclusion criteria
-
(1)
The illness does not fall under the category of chronic respiratory disease;
-
(2)
Inaccessible study data;
-
(3)
non-RCTs, such as observational studies, case series and reviews.
Retrieval strategies
According to the PICOS principal, we adopted mesh terms and free keywords in the search strategy.
-
1.
Population (P): Patients diagnosed with CRD such as COPD, bronchiectasis, and interstitial lung disease.
-
2.
Intervention (I): remote pulmonary rehabilitation, such as telemedicine video consultation, Virtual Autonomous Physiotherapist Agent, video-guided exercises, etc.
-
3.
Comparison (C): standard care (Traditional exercise rehabilitation does not rely on remote technology).
-
4.
Outcome (O): 6-min walk test, St. George’s Respiratory Questionnaire (SGRQ), modified Medical Research Council Dyspnea Scale (mMRC), COPD Assessment Test (CAT), Hospital Anxiety and Depression Scale (HADS), and pulmonary function tests.
-
5.
Study design (S): randomized clinical trials (RCTs).
Computer searches were conducted in The Cochrane Library, MEDLINE, Web of Science, and Embase databases for studies on remote pulmonary rehabilitation since the establishment of the databases to November 14, 2023. English search Medical Subject Headings included: “Telemedicine”[MeSH Terms] AND (“Lung Diseases, Interstitial”[MeSH Terms] OR “Bronchiectasis”[MeSH Terms] OR “Pulmonary disease, chronic obstructive”[MeSH Terms]). The detailed search strategy is provided in Supplementary Material 1.
Literature screening and data extraction
Two reviewers rigorously searched the literature according to the inclusion and exclusion criteria. All identified studies were managed using Endnote software version X9, with the retrieved documents imported into EndNote X9. Duplicate publications and non-English literature were excluded, and studies preliminarily meeting the criteria were screened based on titles or abstracts, with their full texts downloaded. Following full-text reading, original studies meeting the requirements for this systematic review were selected. Information was extracted from the literature and cross-checked, and units of measurement were standardized. In cases of disagreement, a third researcher was consulted for collective decision-making. Extracted information primarily included titles, first authors, publication years, countries, study types, sample sizes, and gender distributions in the intervention and control groups, intervention methods, intervention durations, and outcome measures.
Assessment of bias risk in included studies
Two researchers independently assessed the risk of bias in the eligible studies using a bias assessment tool recommended in the Cochrane Handbook for Systematic Reviews of Interventions version 6.3, Chapter 8: Assessing risk of bias in a randomized trial, the Cochrane risk-of-bias tool for randomized trials (RoB 2), and the results were cross-validated. The risk of bias assessment involved the following seven domains: generation of random sequence (selection bias), allocation concealment (selection bias), blinding of participants and operators (performance bias), and blinding of outcomes assessment (detection bias), integrity of outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias (other bias).
Statistical methods
The meta-analysis was performed with RevMan (Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The magnitude of the effect of each study was calculated by the weighted mean difference (WMD) of the 95% confidence interval (CI) briefly. A p-value of < 0.05 was considered statistically significant unless otherwise specified. In addition, the heterogeneity was quantified using the Q-test and the I2 statistic. When p > 0.1 and I2 < 50%, a fixed-effect model was applied; otherwise, a random-effects model was used. If the heterogeneity was high, further analysis of the heterogeneity sources was performed.
Results
Literature search results
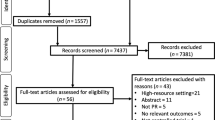
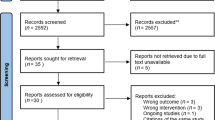
A total of 8893 articles were identified through database searches. After importing the retrieved literature into EndNote X9, 3468 duplicate articles were removed. Following the screening of titles and abstracts, 4404 irrelevant articles were excluded. Subsequently, 994 articles that did not meet the criteria were removed, resulting in the inclusion of 21 articles. The literature screening process and results are shown in Fig. 1.
Basic characteristics of included studies
A total of 21 [14,15,16, 4, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] articles involving 3030 study participants were included, comprising 1509 individuals in the experimental group and 1521 individuals in the control group. The experimental group received telerehabilitation, mainly including telemedicine video consultation, Virtual Autonomous Physiotherapist Agent, video-guided exercises, etc. While the control group received standard care. 6WMD, SGRQ and mMRC are the main outcome indicators used in this study to measure patient improvement. All RCT intervention-related literature was in English. The basic characteristics of the included studies are outlined in Table 1.
Assessment of bias risk in included studies
The quality of the included studies was assessed using the ROB2 tool recommended by Cochrane. Among the 21 trials, the majority of the literature described the randomization process, including whether blinding was utilized, such as through computer-generated random numbers or randomization tables. However, due to the nature of the intervention and certain outcomes (such as self-reported quality of life), there are indeed some biases that cannot be entirely avoided. To more accurately reflect the risk of bias, we considered the specific outcomes in our assessment and performed a detailed analysis for each included randomized controlled trial. Figure 2 presents the risk of bias summary for each trial.
Meta-analysis results
Meta-analysis results of 6MWD
The intervention effect of telerehabilitation on CRD was reported in 14 [14,15,16,17,18,19,20,21,22,23,24,25,26, 4] studies, comprising 638 participants in the experimental group and 654 participants in the control group. Using a fixed-effects model (I2 = 45%, P = 0.02) for effect size pooling, the analysis revealed that compared to conventional rehabilitation in the control group, telerehabilitation in the experimental group demonstrated a significant improvement in outcomes at ≤ 6 months post-intervention [WMD = 7.52, 95%CI (2.09, 12.94)]( See Fig. 3). However, when > 6 months [WMD = 12.89, 95%CI (-0.37, 26.14)], there was no statistically significant difference between the experimental and control groups.
Meta-analysis results of mMRC
The intervention effect of telerehabilitation on CRD was reported in 8 [14, 15, 21, 4, 23,24,25, 28] studies using mMRC, comprising 497 participants in the experimental group and 587 participants in the control group. Employing a fixed-effects model (I2 = 2%, P = 0.43) for effect size pooling, the analysis revealed that compared to conventional rehabilitation in the control group, telerehabilitation in the experimental group showed significant improvement in outcomes both at ≤ 6 months post-intervention [WMD = -0.29, 95%CI (-0.41, -0.18)] and > 6 months [WMD = -0.38, 95%CI (-0.56, -0.21)]. (shown in Fig. 4).
Meta-analysis results of SGRQ
Group discussions were conducted on the SGRQ based on Activity score, impact score, and symptom score. In 6 [16, 20, 21, 30,31,32] studies reporting Activity score, telerehabilitation intervention effects on CRD were examined, with 472 participants in the intervention group and 445 participants in the control group. Using a fixed-effects model (I2 = 0%, P = 0.43) for effect size pooling, the analysis results revealed that compared to conventional rehabilitation in the control group, telerehabilitation in the intervention group demonstrated a significant improvement in outcomes at ≤ 6 months post-intervention [WMD = -1.71, 95%CI (-2.66, -0.76)] (See Fig. 5). However, when > 6 months [WMD = -2.60, 95%CI (-6.00, 0.80)], no statistically significant difference between the intervention and control groups was noticed.
In 6 [16, 20, 21, 30,31,32]studies reporting Impact score, the intervention effects of telerehabilitation on CRD were examined, with 449 participants in the intervention group and 427 participants in the control group. Using a fixed-effects model (I2 = 0%, P = 0.75) for effect size pooling, the analysis results indicated that compared to conventional rehabilitation in the control group, telerehabilitation in the intervention group demonstrated a significant improvement in outcomes at ≤ 6 months post-intervention [WMD = -1.26, 95%CI (-2.15, -0.38)] (See Fig. 6). However, when > 6 months [WMD = -0.69, 95%CI (-3.09, 1.70)], there was no statistically significant difference between the intervention and control groups.
In 6 [16, 20, 21, 30,31,32] studies reporting Symptom score, the intervention effects of telerehabilitation on CRD were examined, with 484 participants in the intervention group and 458 participants in the control group. Employing a fixed-effects model (I2 = 0%, P = 0.81) for effect size pooling, the analysis results demonstrated that compared to conventional rehabilitation in the control group, telerehabilitation in the intervention group exhibited a significant improvement in outcomes at ≤ 6 months post-intervention [WMD = -2.05, 95%CI (-3.05, -1.05)] (See Fig. 7). However, when > 6 months [WMD = -1.66, 95%CI (-5.02, 1.71)], there was no statistically significant difference between the intervention and control groups.
Meta-analysis results of CAT
In 7 [18, 4, 19, 22, 24, 26, 27] studies reporting CAT, the intervention effects of telerehabilitation on CRD were examined, with 309 participants in the intervention group and 396 participants in the control group. Utilizing a random-effects model (I2 = 56%, P = 0.02) for effect size pooling, the analysis results indicated that compared to conventional rehabilitation in the control group, telerehabilitation in the intervention group exhibited a significant improvement in outcomes at ≤ 6 months post-intervention [WMD = -1.77, 95%CI (-3.52, -0.02)] (See Fig. 8). However, when > 6 months [WMD = -1.39, 95%CI (-3.83, 1.05)], there was no statistically significant difference between the intervention and control groups.
Meta-analysis results of pulmonary function
Subgroup analysis of Pulmonary function was conducted based on FEV1% predicted and FEV1/FVC (%). In 3 [17, 23, 33] studies reporting FEV1% predicted, the intervention effects of telerehabilitation on CRD were examined, with 127 participants in the intervention group and 107 participants in the control group. Employing a fixed-effects model (I2 = 51%, P = 0.13) for effect size pooling, the analysis results showed that there was no statistically significant difference between the intervention and control groups in terms of FEV1% predicted [WMD = 2.19, 95%CI (-0.55, 4.93)] (See Fig. 9).
In 2 [17, 33] studies reporting FEV1/FVC (%), the intervention effects of telerehabilitation on CRD were examined, with 97 participants in the intervention group and 77 participants in the control group. Employing a fixed-effects model (I2 = 0%, P = 0.63) for effect size pooling, the analysis results indicated that there was no statistically significant difference between the intervention and control groups in terms of FEV1/FVC (%) [WMD = 2.26, 95%CI (-1.07, 5.59)] (See Fig. 9).
Meta-analysis results of HADS
In 8 [19, 22, 25, 26, 29,30,31,32] studies reporting HADS, the intervention effects of telerehabilitation on CRD were examined, with 633 participants in the intervention group and 668 participants in the control group. Utilizing a fixed-effects model (I2 = 0%, P = 0.55) for effect size pooling, the analysis results indicated that compared to conventional rehabilitation in the control group, telerehabilitation in the intervention group exhibited a significant improvement in outcomes at ≤ 6 months post-intervention [WMD = -0.44, 95%CI (-0.86, -0.03)] (See Fig. 10). However, when > 6 months [WMD = -0.21, 95%CI (-0.69, 0.27)], there was no statistically significant difference between the intervention and control groups.
Publication bias and sensitivity analysis
Publication bias analysis was conducted for each included indicator using a funnel plot to visually display publication bias. Egger’s test was utilized to analyze the funnel plot, with a p-value > 0.05 indicating the absence of publication bias. Egger’s test revealed a p-value of 0.019 for the 6MWD indicator (Table 2), indicating the presence of publication bias among the studies. Therefore, for indicators exhibiting publication bias, a trim-and-fill method was employed for further analysis. After incorporating six additional studies into the model to achieve funnel plot symmetry, the combined effect size for the 6MWD indicator was 5.836, with a 95% confidence interval of (0.925, 10.746) (Table 3).
Sensitivity analysis was performed by individually excluding each study from the meta-analysis to assess the stability and reliability of the results. The sensitivity analysis results indicated that the meta-analysis results were stable and reliable.
Discussion
Data indicates that approximately 3 million people die from COPD each year, with COPD projected to become the third leading cause of death worldwide by 2020 [34]. In 2013, the American Thoracic Society and the European Respiratory Society introduced a home-based pulmonary rehabilitation program aimed at providing pulmonary rehabilitation services for patients with COPD in the home environment. This pulmonary rehabilitation program involves comprehensive assessment of patients’ conditions and implementing integrated intervention measures based on personalized treatment [35]. However, due to issues such as resource shortages, high costs, and inconvenient transportation, out-of-hospital patients have lower compliance with pulmonary rehabilitation [36]. Remote home-based pulmonary rehabilitation, based on multimedia technology combined with computer and network technology, integrates with medical technology in large hospitals to provide remote online rehabilitation medical information and technical services. This form of rehabilitation service enables COPD patients to effectively integrate rehabilitation into their daily lives, while also reducing economic burdens to some extent, bringing certain benefits to patients [37]. It overcomes geographical limitations, to some extent addressing the shortage of medical resources in remote areas, further improving and enhancing the level of rehabilitation services in major cities, and greatly promoting the development of medical and healthcare industries. Currently, remote technology has been widely used to provide rehabilitation services for patients with COPD [22], asthma [38], heart failure [39], stroke [40], and other conditions.
A meta-analysis was performed, incorporating data 21 RCTs, to evaluate the effectiveness of remote pulmonary rehabilitation interventions for CRD. The study enrolled 1521 patients in the control group and 1509 patients in the intervention group. Primary outcome measures encompassed 6WMD, SGRQ, mMRC, CAT, HADS, and pulmonary function.
In the short term (≤ 6 months) observation, significant improvements were observed in 6WMD, mMRC, SGRQ, and CAT. The 6WMD reflected patients’ daily activity capacity, mMRC assessed the severity of dyspnea, SGRQ evaluated health status and quality of life, and CAT assessed disease severity and quality of life. However, it is important to note that the minimum clinically significant difference for the 6-min walking test is 30 m. Therefore, despite the statistical significance, the improvement observed does not reach the threshold for clinical relevance. The results of this study are consistent with previous research that has demonstrated the benefits of remote pulmonary rehabilitation in enhancing patients’ health status and quality of life. For example, Michaelchuk et al. (2022) [10] found similar improvements in CAT and mMRC following remote pulmonary rehabilitation in patients with COPD. These findings suggested that remote pulmonary rehabilitation interventions can substantially enhance patients’ activity capacity, alleviate dyspnea, and improve health status and quality of life in the short term. This improvement may be attributed to personalized rehabilitation plans provided by remote pulmonary rehabilitation and effective rehabilitation training facilitated by regular monitoring and guidance.
Notably, in long-term follow-up (> 6 months), while improvements in 6WMD, mMRC, SGRQ, and CAT still existed between the intervention and control groups, only the difference in mMRC was statistically significant. This may be due to increased loss to follow-up, reduced sample size, or decreased compliance of patients with remote pulmonary rehabilitation over the long term. Therefore, further long-term studies are needed to determine the long-term effects of remote pulmonary rehabilitation.
Furthermore, we conducted an evaluation using the widely adopted HADS, which is designed to assess anxiety and depression across various illnesses. The findings revealed significant improvements in HADS scores within ≤ 6 months post-intervention, indicating that telerehabilitation not only enhances the health status of patients but also ameliorates anxiety and depression among CRD patients, thereby enhancing their overall quality of life. However, over observation periods exceeding 6 months, there were no statistically significant differences observed between the intervention and control groups. This could be attributed to factors such as the chronic nature of airflow limitation in the disease, prolonged and slow disease progression, and decreased treatment adherence. It’s worth noting that due to the limited number of studies, we aggregated the anxiety and depression subscales for analysis. Future research is warranted to delve into separate analyses of anxiety and depression.
In terms of pulmonary function, this study conducted subgroup analysis based on FEV1% predicted and FEV1/FVC (%) and included a total of 5 studies reporting changes in pulmonary function outcomes. The results indicated no statistically significant differences between the intervention and control groups, which is consistent with the findings of Du et al. [41]. Given the possibility of insufficient sample sizes in the included studies, it is hoped that future research will involve long-term follow-up of these indicators to provide robust evidence for confirming the long-term effects of remote pulmonary rehabilitation.
Compared to previously published meta-analyses, this study’s strength lies in providing remote real-time interventions according to pulmonary rehabilitation measures and conducting statistical analyses of outcome indicators. It investigated the intervention effects of different time periods on CRD, thereby ensuring the research results are more rigorous and scientific. Additionally, it analyzed the anxiety and depression levels of patients, providing a comprehensive evaluation of the intervention effects of remote pulmonary rehabilitation on CRD. This offers more comprehensive evidence-based support for the real needs of CRD patients for remote pulmonary rehabilitation and provides targeted remote pulmonary rehabilitation services. The evidence from this study supports the effectiveness of remote pulmonary rehabilitation in improving alleviate dyspnea, and improve health status and quality of life for CRD patients, particularly in the short term. While the evidence is strong for COPD, more research is needed to determine the effectiveness of remote pulmonary rehabilitation in other respiratory diseases. To implement remote pulmonary rehabilitation at a national level in all pulmonary rehabilitation programs, it is essential to provide training for health personnel in the use of technological tools that enable them to deliver tailored interventions to respiratory patients. This training should encompass a range of professionals, including doctors, nurses, and physiotherapists, and should focus on familiarizing them with remote monitoring systems, online consultation platforms, and digital rehabilitation programs. Additionally, ongoing education and support should be provided to ensure that health personnel are proficient in utilizing these tools effectively and that they remain up-to-date with advancements in remote healthcare technology.
However, this study also has certain limitations. There were differences among the included study populations and baseline values, leading to higher heterogeneity in some positive results. Additionally, some studies did not describe allocation concealment and blinding methods, potentially introducing selection bias, implementation bias, and measurement bias. Moreover, the number of studies with long-term follow-up on efficacy was limited, possibly resulting in low test power. Furthermore, the economic benefits and costs associated with implementing telerehabilitation were not assessed in the included studies. It is hoped that future research will conduct more double-blind randomized controlled trials on the intervention effects of remote pulmonary rehabilitation in CRD, expand the sample size, extend the follow-up period, and observe outcome indicators comprehensively and with more standardized data, to provide more scientific evidence for the effectiveness and feasibility of remote pulmonary rehabilitation in CRD.
Conclusion
The meta-analysis indicates that utilizing telerehabilitation therapy can improve respiratory function and mental health status in the short term, ultimately enhancing the quality of life for CRD patients. However, further evidence from more high-quality, large-sample randomized controlled trials is needed to establish the long-term effectiveness of this rehabilitation approach.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Abbreviations
- CRD:
-
Chronic respiratory disease
- COPD:
-
Chronic Obstructive Pulmonary Disease
- CPH:
-
China Pulmonary Health
- TR:
-
Telerehabilitation
- PROSPERO:
-
Preferred Reporting Items for Systematic Review and Meta-Analyses
- 6MWD:
-
6-Minute walk distance
- SGRQ:
-
St. George’s Respiratory Questionnaire
- mMRC:
-
Modified Medical Research Council Dyspnea Scale
- CAT:
-
COPD Assessment Test
- HADS:
-
Hospital Anxiety and Depression Scale
- CI:
-
Confidence interval
- FEV1:
-
Forced expiratory volume in the first one second
- FVC:
-
Forced vital capacity
- RCT:
-
Randomized controlled trial
References
GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–96. https://doi.org/10.1016/S2213-2600(20)30105-3.
Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–17.
Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–58.
Zanaboni P, Dinesen B, Hoaas H, Wootton R, Burge AT, Philp R, et al. Long-term telerehabilitation or unsupervised training at home for patients with chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2023;207(7):865–75.
Nici L. Pulmonary rehabilitation after a chronic obstructive pulmonary disease exacerbation: impact on readmission risk in a real-world setting. Am J Respir Crit Care Med. 2021;204(9):1005–6.
Song X, Hallensleben C, Zhang W, Jiang Z, Shen H, Gobbens RJJ, et al. Blended self-management interventions to reduce disease burden in patients with chronic obstructive pulmonary disease and asthma: systematic review and meta-analysis. J Med Internet Res. 2021;23(3):e24602.
Song CY, Liu X, Wang YQ, Cao HP, Yang Z, Ma RC, et al. Effects of home-based telehealth on the physical condition and psychological status of patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Int J Nurs Pract. 2023;29(3):e13062.
Lee AYL, Wong AKC, Hung TTM, Yan J, Yang S. Nurse-led telehealth intervention for rehabilitation (Telerehabilitation) among community-dwelling patients with chronic diseases: systematic review and meta-analysis. J Med Internet Res. 2022;24(11):e40364.
Timoteo EF, Silva DF, Oliveira TM, José A, Malaguti C. Real-time telerehabilitation for chronic respiratory disease and post-COVID-19: A systematic review and meta-analysis. J Telemed Telecare. 024:1357633X241241572. https://doi.org/10.1177/1357633X241241572.
Michaelchuk W, Oliveira A, Marzolini S, Nonoyama M, Maybank A, Goldstein R, et al. Design and delivery of home-based telehealth pulmonary rehabilitation programs in COPD: A systematic review and meta-analysis. Int J Med Inform. 2022;162:104754.
Ora J, Prendi E, Attinà ML, Cazzola M, Calzetta L, Rogliani P. Efficacy of respiratory tele-rehabilitation in COPD patients: Systematic review and meta-analysis. Monaldi Arch Chest Dis. 2022;92(4):10.4081/monaldi.2022.2105.
Cox NS, Dal Corso S, Hansen H, McDonald CF, Hill CJ, Zanaboni P, et al. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev. 2021;1(1):Cd013040.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Holland AE, Mahal A, Hill CJ, Lee AL, Burge AT, Cox NS, et al. Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax. 2017;72(1):57–65.
Lahham A, McDonald CF, Moore R, Cox NS, Rawlings S, Nichols A, et al. The impact of home-based pulmonary rehabilitation on people with mild chronic obstructive pulmonary disease: A randomised controlled trial. Clin Respir J. 2020;14(4):335–44.
Wong EY, Jennings CA, Rodgers WM, Selzler AM, Simmonds LG, Hamir R, et al. Peer educator vs. respiratory therapist support: which form of support better maintains health and functional outcomes following pulmonary rehabilitation? Patient Educ Couns. 2014;95(1):118–25.
Oh EG. The effects of home-based pulmonary rehabilitation in patients with chronic lung disease. Int J Nurs Stud. 2003;40(8):873–9.
Cameron-Tucker HL, Wood-Baker R, Joseph L, Walters JA, Schüz N, Walters EH. A randomized controlled trial of telephone-mentoring with home-based walking preceding rehabilitation in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1991–2000.
Hansen H, Bieler T, Beyer N, Kallemose T, Wilcke JT, Østergaard LM, et al. Supervised pulmonary tele-rehabilitation versus pulmonary rehabilitation in severe COPD: a randomised multicentre trial. Thorax. 2020;75(5):413–21.
Cerdán-de-Las-Heras J, Balbino F, Løkke A, Catalán-Matamoros D, Hilberg O, Bendstrup E. Effect of a new tele-rehabilitation program versus standard rehabilitation in patients with chronic obstructive pulmonary Disease. J Clin Med. 2021;11(1):1.
de Sousa Pinto JM, Martín-Nogueras AM, Calvo-Arenillas JI, Ramos-González J. Clinical benefits of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2014;34(5):355–9.
Tsai LL, McNamara RJ, Moddel C, Alison JA, McKenzie DK, McKeough ZJ. Home-based telerehabilitation via real-time videoconferencing improves endurance exercise capacity in patients with COPD: The randomized controlled TeleR Study. Respirology. 2017;22(4):699–707.
Wang L, Sun B, Cui H, Wang W, Ren Q, Sun Y, et al. Long-term effects of home-based pulmonary rehabilitation on idiopathic interstitial pneumonia patients. All Life. 2021;14(1):181–6.
Vasilopoulou M, Papaioannou AI, Kaltsakas G, Louvaris Z, Chynkiamis N, Spetsioti S, et al. Home-based maintenance tele-rehabilitation reduces the risk for acute exacerbations of COPD, hospitalisations and emergency department visits. Eur Respir J. 2017;49(5):1602129.
Cox NS, McDonald CF, Mahal A, Alison JA, Wootton R, Hill CJ, et al. Telerehabilitation for chronic respiratory disease: a randomised controlled equivalence trial. Thorax. 2022;77(7):643–51.
Godtfredsen N, Frølich A, Bieler T, Beyer N, Kallemose T, Wilcke T, et al. 12-months follow-up of pulmonary tele-rehabilitation versus standard pulmonary rehabilitation: a multicentre randomised clinical trial in patients with severe COPD. Respir Med. 2020;172:106129.
Billington J, Coster S, Murrells T, Norman I. Evaluation of a Nurse-Led educational telephone intervention to support self-management of patients with chronic obstructive pulmonary disease: a randomized feasibility study. COPD. 2015;12(4):395–403.
MD RB, Hoult J, McEvoy C, Clark M. Promoting COPD wellness through remote monitoring and health coaching: A Randomized Study. 2022.
Horton EJ, Mitchell KE, Johnson-Warrington V, Apps LD, Sewell L, Morgan M, Taylor RS, Singh SJ. Comparison of a structured home-based rehabilitation programme with conventional supervised pulmonary rehabilitation: a randomised non-inferiority trial. Thorax. 2018;73(1):29–36. https://doi.org/10.1136/thoraxjnl-2016-208506.
Walters J, Cameron-Tucker H, Wills K, Schüz N, Scott J, Robinson A, et al. Effects of telephone health mentoring in community-recruited chronic obstructive pulmonary disease on self-management capacity, quality of life and psychological morbidity: a randomised controlled trial. BMJ Open. 2013;3(9):e003097.
Jolly K, Sidhu MS, Hewitt CA, Coventry PA, Daley A, Jordan R, et al. Self management of patients with mild COPD in primary care: randomised controlled trial. bmj. 2018;361.
Schou L, Østergaard B, Rydahl-Hansen S, Rasmussen LS, Emme C, Jakobsen AS. A randomised trial of telemedicine-based treatment versus conventional hospitalisation in patients with severe COPD and exacerbation–effect on self-reported outcome. J Telemed Telecare. 2013;19(3):160–5. https://doi.org/10.1177/1357633X13483255.
Li Y, Feng J, Li Y, Jia W, Qian H. A new pulmonary rehabilitation maintenance strategy through home-visiting and phone contact in COPD. Patient preference and adherence. 2018:97–104.
Ding H, Karunanithi M, Kanagasingam Y, Vignarajan J, Moodley Y. A pilot study of a mobile-phone-based home monitoring system to assist in remote interventions in cases of acute exacerbation of COPD. J Telemed Telecare. 2014;20(3):128–34. https://doi.org/10.1177/1357633X14527715.
Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American thoracic society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64.
Wadell K, Janaudis Ferreira T, Arne M, Lisspers K, Ställberg B, Emtner M. Hospital-based pulmonary rehabilitation in patients with COPD in Sweden–a national survey. Respir Med. 2013;107(8):1195–200.
Zanaboni P, Dinesen B, Hjalmarsen A, Hoaas H, Holland AE, Oliveira CC, et al. Long-term integrated telerehabilitation of COPD Patients: a multicentre randomised controlled trial (iTrain). BMC Pulm Med. 2016;16(1):126.
Katwa U, Rivera E. Asthma Management in the Era of Smart-Medicine: Devices, Gadgets. Apps and Telemedicine Indian J Pediatr. 2018;85(9):757–62.
Bernocchi P, Vitacca M, La Rovere MT, Volterrani M, Galli T, Baratti D, et al. Home-based telerehabilitation in older patients with chronic obstructive pulmonary disease and heart failure: a randomised controlled trial. Age Ageing. 2018;47(1):82–8.
Dodakian L, McKenzie AL, Le V, See J, Pearson-Fuhrhop K, Burke Quinlan E, et al. A home-based telerehabilitation program for patients with stroke. Neurorehabil Neural Repair. 2017;31(10–11):923–33.
Du Y, Lin J, Wang X, Zhang Y, Ge H, Wang Y, et al. Early pulmonary rehabilitation in acute exacerbation of chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. COPD. 2022;19(1):69–80.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: YD; Writing—original draft preparation: YD; Methodology: YD, HH, YCZ, NH, MS; Resources: YD, HH, YCZ; Formal analysis and investigation: YD, NH, MS; Supervision: HL; Writing—review and editing: All authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dai, Y., Huang, H., Zhang, Y. et al. The effects of telerehabilitation on physiological function and disease symptom for patients with chronic respiratory disease: a systematic review and meta-analysis. BMC Pulm Med 24, 305 (2024). https://doi.org/10.1186/s12890-024-03104-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-03104-8